A Non-Conformance Report Document Sample for Quality Management provides a structured template to identify, document, and address deviations from established quality standards. This sample helps organizations systematically track non-conformities, analyze root causes, and implement corrective actions to improve processes. Utilizing such a document ensures consistent quality control and enhances overall compliance with industry regulations.
Manufacturing Non-Conformance Report Template
A
Manufacturing Non-Conformance Report Template document is a standardized form used to record deviations from production specifications or quality standards during the manufacturing process. It captures critical information such as the nature of the non-conformance, root cause analysis, corrective actions, and responsible personnel to ensure effective resolution and prevention of recurrence. This template facilitates consistent documentation, improves traceability, and supports compliance with quality management systems like ISO 9001.
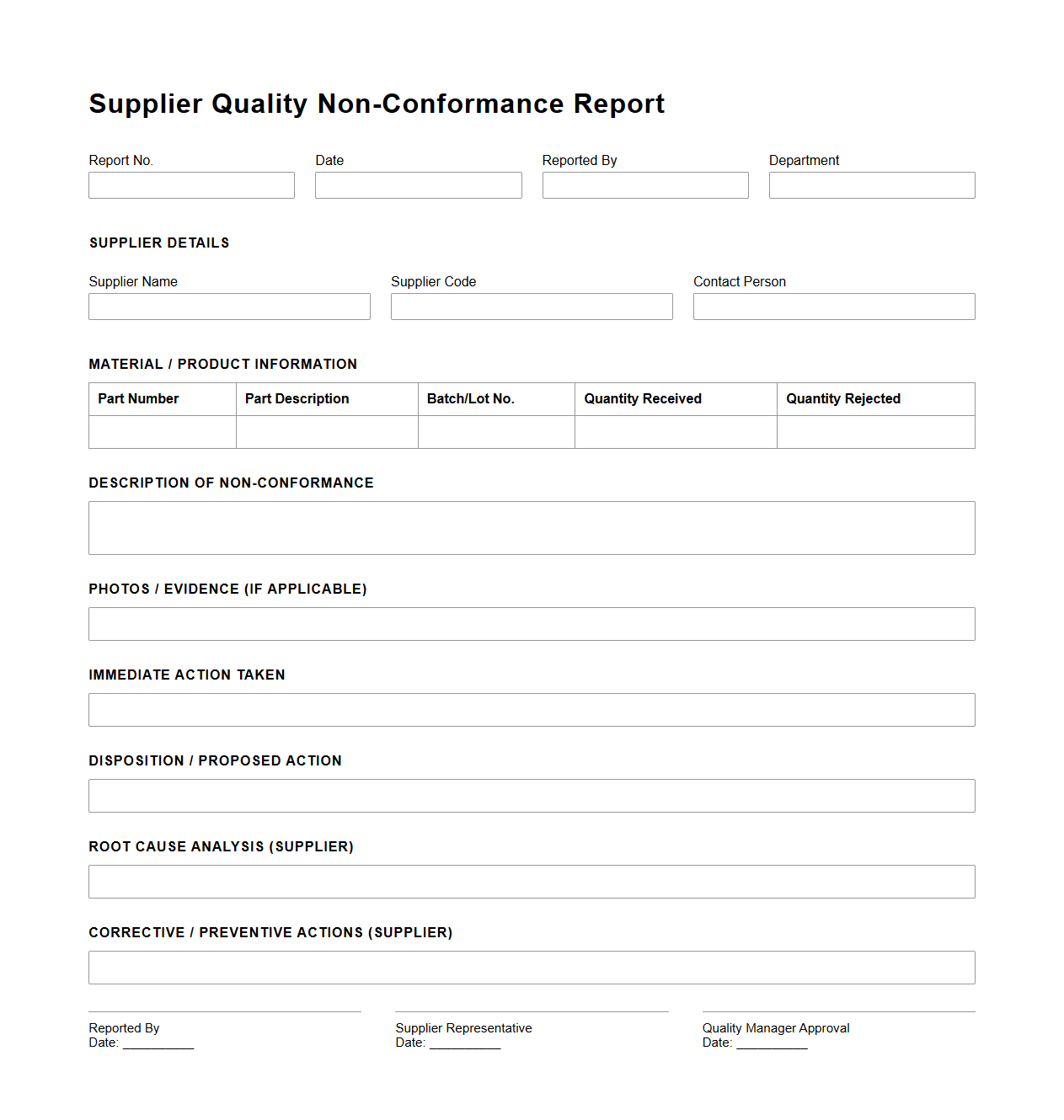
Supplier Quality Non-Conformance Report Example
A
Supplier Quality Non-Conformance Report (NCR) Example document serves as a formal record identifying instances where supplied materials or products fail to meet established quality standards or specifications. This document details the nature of the non-conformance, including relevant data such as part numbers, batch codes, defect descriptions, and any immediate containment actions taken. It is essential for tracking supplier performance, facilitating corrective actions, and maintaining supply chain quality assurance.
Internal Audit Non-Conformance Report Format
The
Internal Audit Non-Conformance Report Format document is a structured template used to record instances where processes or practices deviate from established standards during an internal audit. It captures detailed information such as the nature of the non-conformance, the affected department or process, evidence collected, and the immediate corrective actions required. This format ensures consistent reporting, facilitates root cause analysis, and supports continuous improvement in quality management systems.
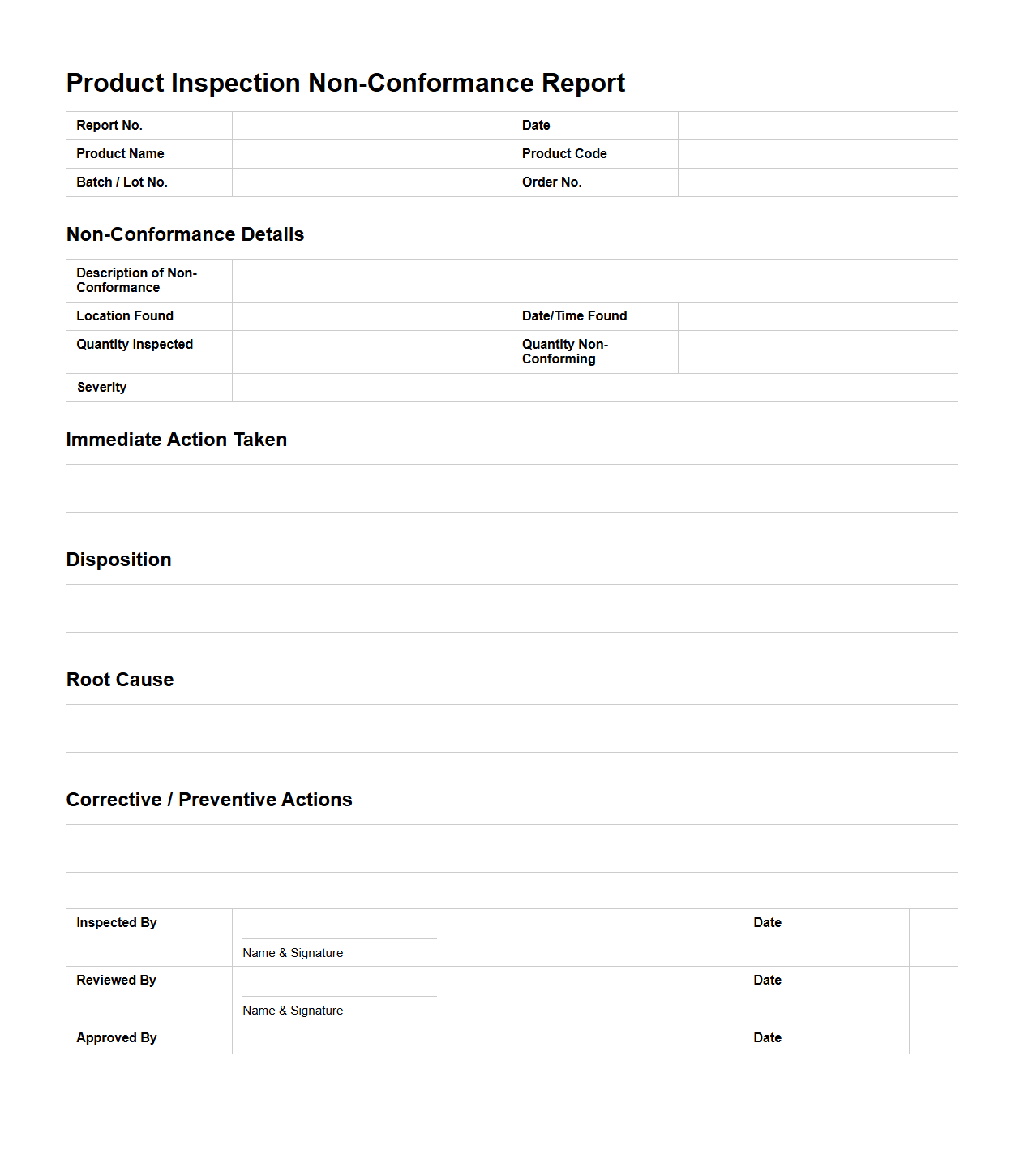
Product Inspection Non-Conformance Report Layout
A
Product Inspection Non-Conformance Report Layout document is a structured template used to record and analyze deviations identified during the product inspection process. It typically includes sections for detailing the non-conforming product, nature of the defect, inspection criteria, root cause analysis, and corrective actions. This document ensures consistent reporting and facilitates continuous quality improvement within manufacturing and quality control environments.
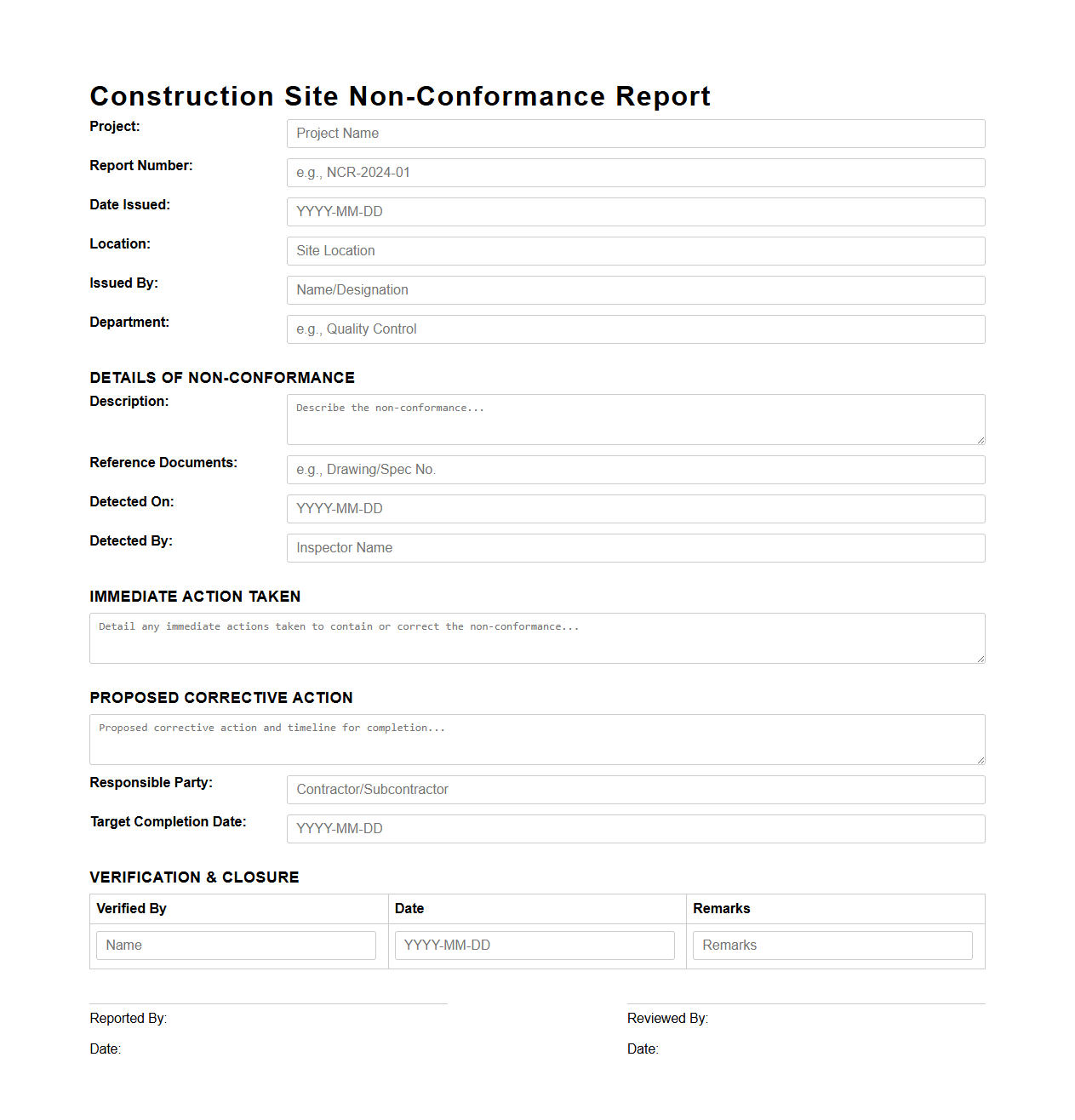
Construction Site Non-Conformance Report Sample
A
Construction Site Non-Conformance Report Sample document serves as a standardized template to record deviations from project specifications, standards, or safety regulations encountered during construction activities. It details the nature of the non-conformance, involved parties, corrective actions required, and timelines for resolution to ensure quality control and compliance. This document is crucial for effective project management, risk mitigation, and maintaining adherence to contractual and regulatory requirements on construction sites.
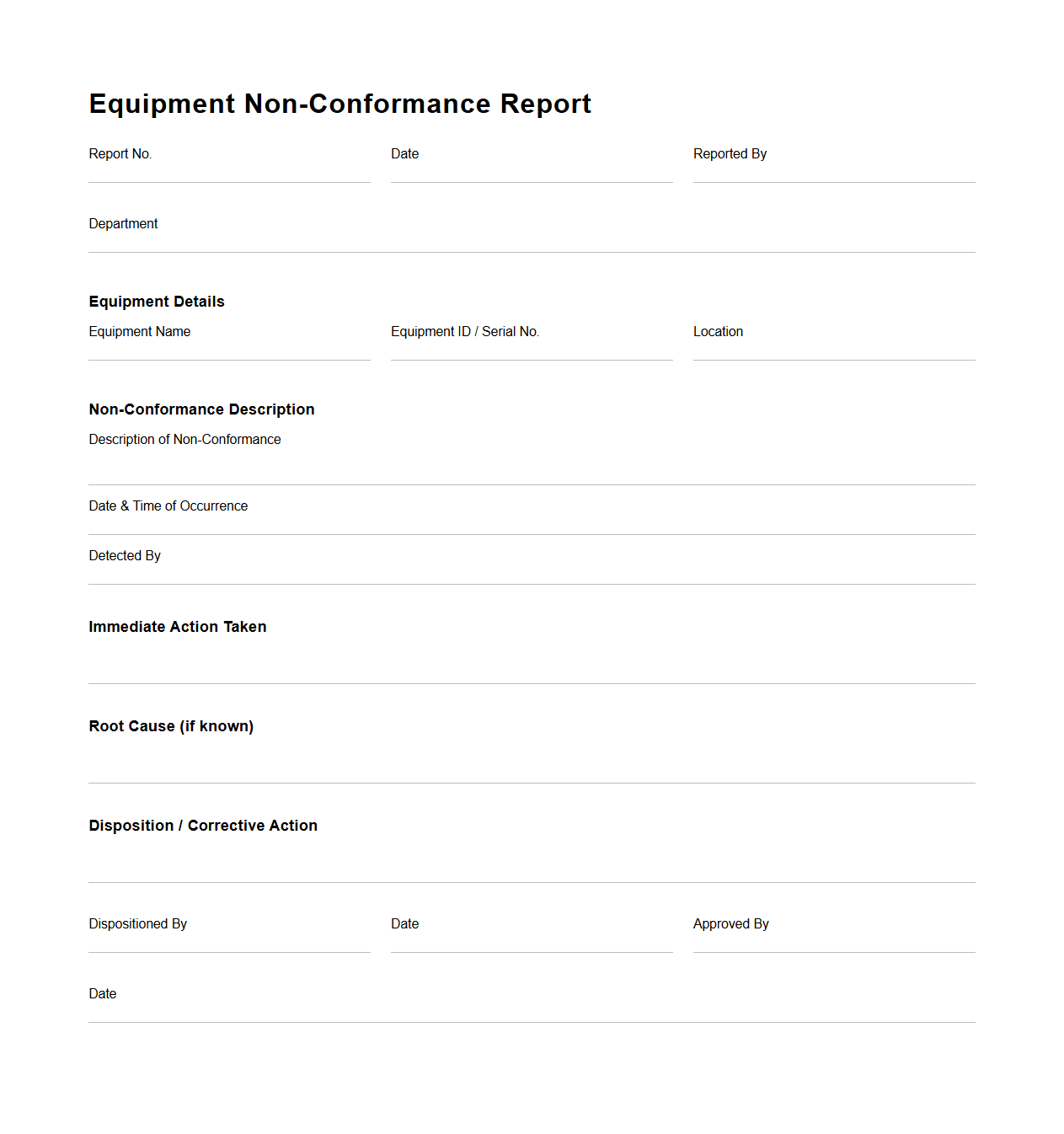
Equipment Non-Conformance Report Document
An
Equipment Non-Conformance Report Document is a formal record used to identify, document, and address deviations or defects in equipment that do not meet specified standards or requirements. This document details the nature of the non-conformance, including the affected equipment, detection method, and potential impact on operations or safety. It serves as a critical tool for quality control, root cause analysis, and corrective action planning within manufacturing and maintenance processes.
Laboratory Non-Conformance Report Form
The
Laboratory Non-Conformance Report Form document is used to systematically identify, document, and address deviations from standard laboratory procedures or quality requirements. It ensures that any non-conformance incidents are recorded accurately, enabling corrective actions to be implemented to maintain compliance with regulatory and quality standards. This form supports continuous improvement by facilitating root cause analysis and preventing recurrence of errors in laboratory operations.
Warehouse Non-Conformance Report Sheet
The
Warehouse Non-Conformance Report Sheet document is a critical tool used to identify and record deviations from established warehouse processes or standards, ensuring effective quality control. It documents specific issues such as damaged goods, incorrect inventory counts, or procedural violations, providing detailed information for corrective actions. This sheet supports continuous improvement by tracking non-conformance trends and facilitating accountability within warehouse operations.
Service Process Non-Conformance Report Template
A
Service Process Non-Conformance Report Template document is used to systematically record and analyze deviations from established service procedures. It helps organizations identify root causes, assess impact, and implement corrective actions to improve service quality. This template ensures consistent documentation for compliance and continuous process enhancement.
Customer Complaint Non-Conformance Report Example
A
Customer Complaint Non-Conformance Report Example document serves as a standardized template to record and analyze instances where a product or service fails to meet customer expectations or contractual requirements. It details the nature of the complaint, identifies root causes, and tracks corrective actions to prevent recurrence, ensuring quality control and customer satisfaction. This report is essential for maintaining compliance with industry standards and improving organizational processes.
What specific criteria trigger the issuance of a Non-Conformance Report in your quality management system?
A Non-Conformance Report (NCR) is initiated when a product, process, or service fails to meet predefined quality standards. This includes deviations from specifications, regulatory requirements, or internal procedures. The report officially documents any identified non-conformities to ensure prompt corrective action.
How are root causes documented and categorized within the Non-Conformance Report?
Root causes are identified through systematic analysis methods such as 5 Whys or Fishbone Diagrams within the NCR. Each cause is documented clearly and categorized by type, such as human error, process failure, or equipment malfunction. This categorization facilitates proper corrective strategies aligned with the underlying issues.
Which stakeholders are required to review and sign off on the Non-Conformance Report?
The NCR must be reviewed and signed by essential stakeholders including Quality Assurance, Production Management, and the involved Process Owner. This multi-level authorization ensures accountability and oversight throughout the corrective action process. Senior management may also be required to approve depending on the severity of the issue.
What timeline is allocated for corrective action closure in each Non-Conformance Report?
The standard timeline for closing corrective actions in an NCR typically ranges from 15 to 30 business days, depending on the complexity of the non-conformance. Extensions can be granted if justified and documented appropriately. Timely resolution is critical to prevent recurrence and maintain compliance.
How is the effectiveness of corrective actions tracked and referenced in subsequent Non-Conformance Reports?
Effectiveness is tracked by monitoring key performance indicators and conducting follow-up audits documented within the NCR system. Subsequent NCRs reference previous corrective actions to ensure continuous improvement and prevent repeated issues. This feedback loop strengthens the overall quality management process.
More Construction Templates