A Compliance Checklist Document Sample for Healthcare Technology serves as a structured guide to ensure organizations meet regulatory requirements and industry standards. It covers critical areas such as data privacy, security protocols, and system interoperability to safeguard patient information and maintain operational integrity. Utilizing this checklist helps streamline audits and supports continuous compliance management within healthcare technology environments.
HIPAA Compliance Assessment Checklist for Healthcare Technology
The
HIPAA Compliance Assessment Checklist for Healthcare Technology is a detailed guide that helps healthcare organizations evaluate their adherence to HIPAA regulations. It includes key criteria such as data protection measures, risk analysis, access controls, and employee training to ensure the confidentiality, integrity, and availability of electronic protected health information (ePHI). This document is essential for identifying gaps in compliance and implementing corrective actions to avoid penalties and safeguard patient information.
Data Security Compliance Checklist for Medical Software
The
Data Security Compliance Checklist for medical software is a comprehensive guide ensuring that healthcare applications meet regulatory standards like HIPAA and GDPR. It outlines essential security protocols, including encryption, access controls, and audit trails, to protect sensitive patient information from breaches. This document is critical for healthcare providers and developers to maintain legal compliance and safeguard medical data integrity.
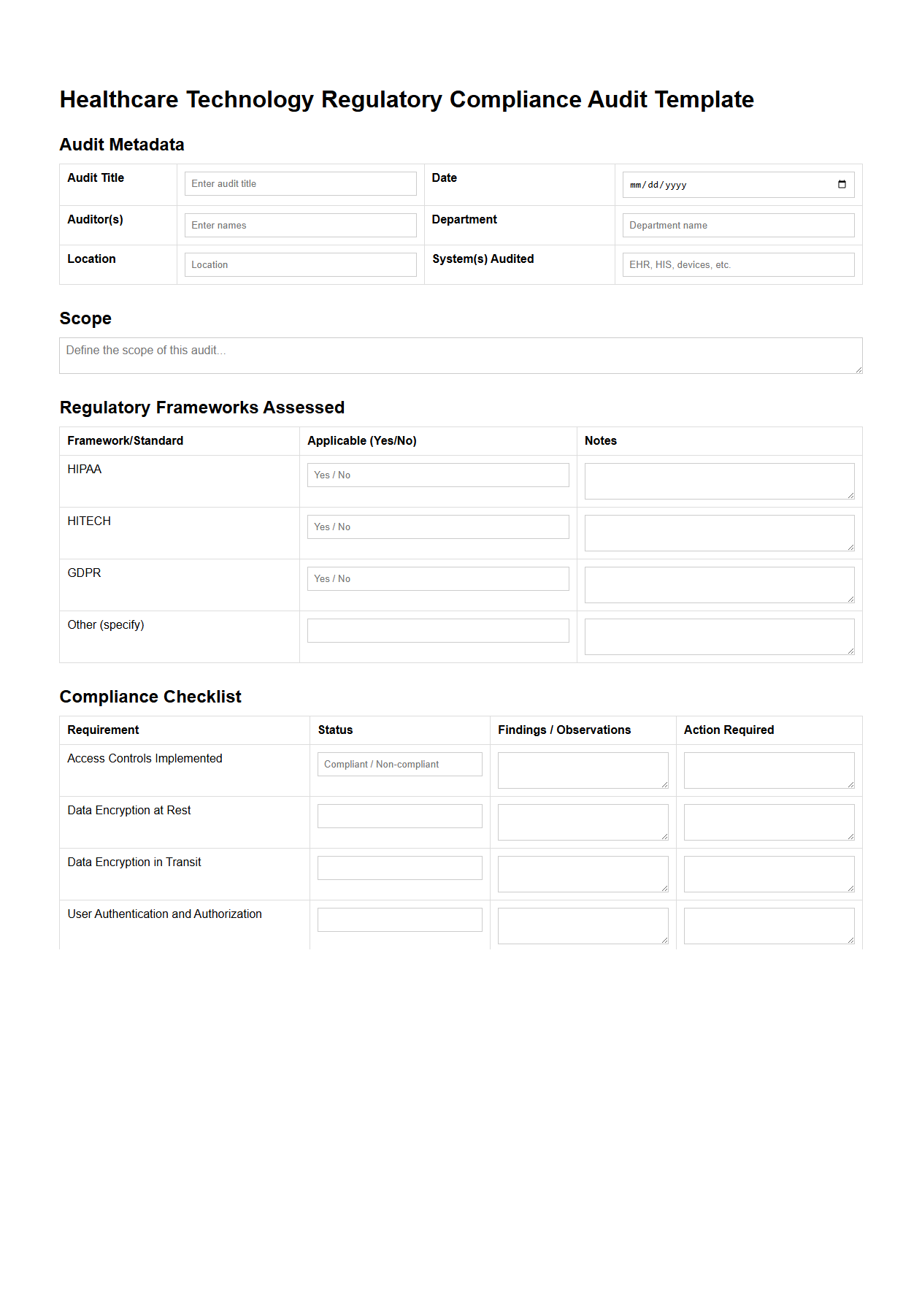
Healthcare Technology Regulatory Compliance Audit Template
A
Healthcare Technology Regulatory Compliance Audit Template document serves as a structured guide to systematically evaluate whether healthcare technology systems adhere to relevant laws, standards, and regulations such as HIPAA, FDA guidelines, and GDPR. It outlines specific audit criteria, checklists, and documentation requirements to ensure that data security, patient privacy, and operational protocols meet compliance mandates. This template is essential for healthcare providers and technology vendors to identify compliance gaps and mitigate risks associated with legal and regulatory breaches.
Patient Data Privacy Compliance Checklist for Digital Health Platforms
The
Patient Data Privacy Compliance Checklist for Digital Health Platforms outlines essential standards and protocols to safeguard sensitive health information in accordance with regulations such as HIPAA and GDPR. It ensures digital health providers implement robust data encryption, secure access controls, and regular privacy audits to maintain confidentiality and integrity of patient data. Following this checklist helps platforms mitigate risks of data breaches and maintain trust with users by adhering to legal and ethical privacy requirements.
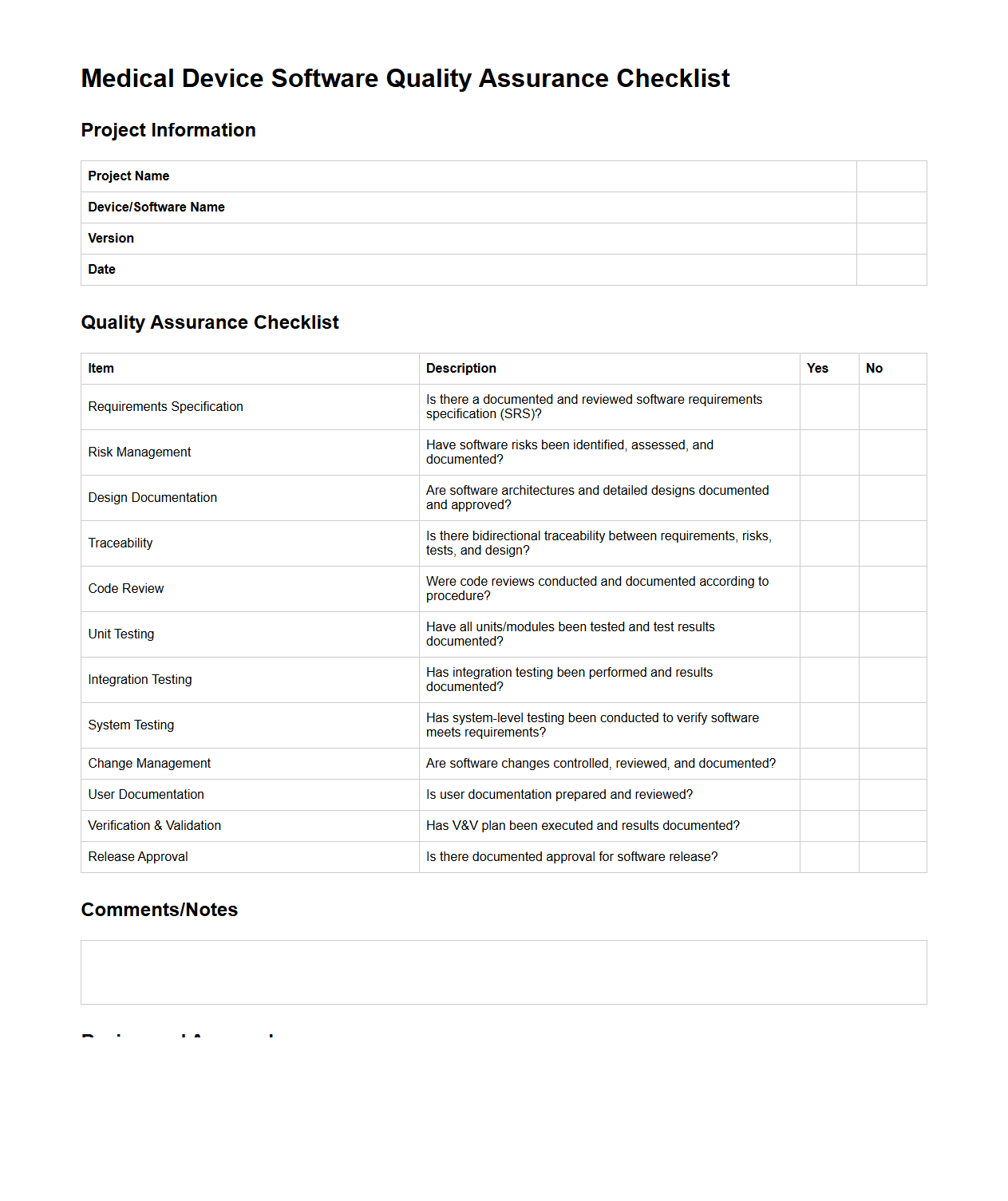
Medical Device Software Quality Assurance Checklist
A
Medical Device Software Quality Assurance Checklist document is a comprehensive tool used to ensure that all regulatory and industry standards for medical device software are met throughout development and deployment. It systematically verifies compliance with quality management systems such as ISO 13485 and FDA 21 CFR Part 820, addressing risk management, validation activities, and documentation control. This checklist helps organizations maintain high software quality, enhance patient safety, and prepare for audits by identifying gaps and verifying proper implementation of quality assurance processes.
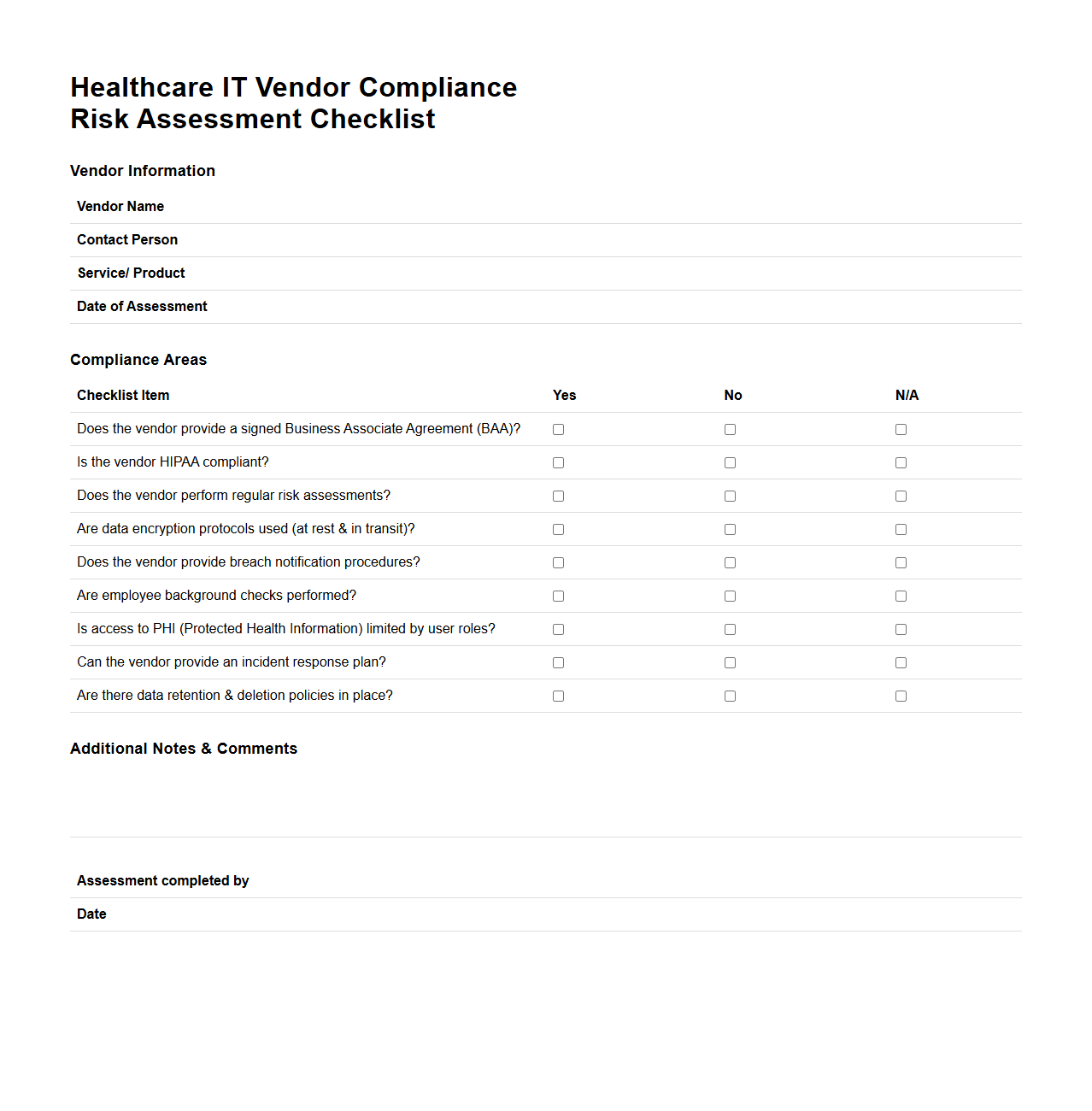
Healthcare IT Vendor Compliance Risk Assessment Checklist
The
Healthcare IT Vendor Compliance Risk Assessment Checklist document serves as a critical tool for evaluating a vendor's adherence to healthcare regulations and standards such as HIPAA and GDPR. It systematically identifies potential risks related to data security, patient privacy, and regulatory compliance, ensuring vendors meet industry-specific requirements. This checklist assists healthcare organizations in mitigating legal and operational risks associated with third-party IT service providers.
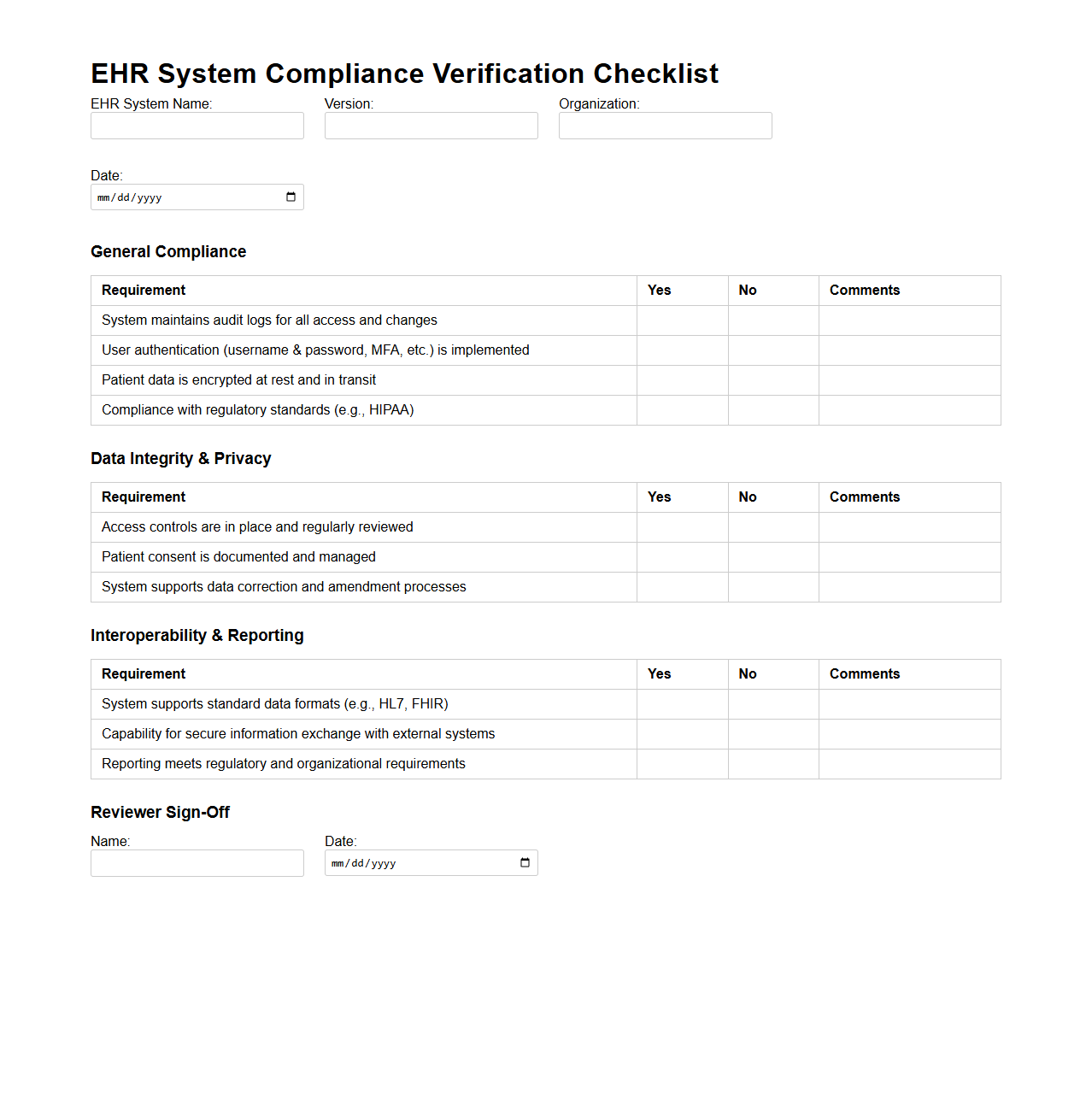
EHR System Compliance Verification Checklist
The
EHR System Compliance Verification Checklist document serves as a critical tool for healthcare organizations to ensure their Electronic Health Record (EHR) systems meet regulatory standards and industry best practices. It systematically verifies key compliance aspects such as data security, patient privacy, interoperability, and audit trail functionalities. This checklist helps identify gaps, facilitating corrective actions to maintain certification and protect sensitive health information.
Telehealth Service Compliance Documentation Checklist
The
Telehealth Service Compliance Documentation Checklist is a comprehensive tool designed to ensure healthcare providers meet regulatory standards and legal requirements in telehealth delivery. It systematically verifies documentation related to patient consent, privacy protocols, licensing, billing accuracy, and technology security measures. Utilizing this checklist helps organizations maintain adherence to healthcare laws, improve service quality, and reduce the risk of non-compliance penalties.
Clinical Data Integration Compliance Checklist
The
Clinical Data Integration Compliance Checklist document serves as a structured guide to ensure that all clinical data integration activities adhere to regulatory standards and industry best practices. It outlines critical compliance requirements for data accuracy, security, interoperability, and patient privacy during the integration process. This checklist helps healthcare organizations systematically verify that clinical information systems align with legal frameworks such as HIPAA and FDA regulations.
Health Information Exchange Compliance Review Checklist
The
Health Information Exchange Compliance Review Checklist document serves as a critical tool for evaluating adherence to regulatory standards in the secure sharing of electronic health information. It outlines specific criteria for ensuring data privacy, interoperability, and accuracy within health information exchange processes. Organizations use this checklist to systematically verify compliance with HIPAA, HITECH, and other relevant healthcare regulations.
What cybersecurity protocols are required in the compliance checklist for healthcare tech documentation?
The compliance checklist must include encryption standards to protect sensitive healthcare data. It also requires robust access control mechanisms to prevent unauthorized system usage. Additionally, regular vulnerability assessments and incident response plans are essential components.
Which specific patient data privacy standards must be referenced in the checklist?
The checklist must explicitly reference the Health Insurance Portability and Accountability Act (HIPAA) for patient data privacy. It should also include compliance with General Data Protection Regulation (GDPR) for international patient information. Moreover, state-specific privacy laws, like California Consumer Privacy Act (CCPA), are vital to include.
How does the compliance document address software interoperability regulations?
The document mandates adherence to Health Level Seven (HL7) standards for seamless data exchange. It emphasizes using APIs that comply with the FHIR (Fast Healthcare Interoperability Resources) framework. This ensures consistent communication across different healthcare technologies.
What audit trail documentation is mandated for healthcare technology processes?
Maintaining detailed audit logs that record user actions and system changes is required by the compliance checklist. The documentation must ensure the traceability of all data access and modifications. These logs are critical for security reviews and forensic investigations.
Are device maintenance and calibration records included in the compliance checklist scope?
Yes, the checklist requires comprehensive device maintenance and calibration records to ensure operational accuracy. Regular documentation helps in meeting regulatory standards and maintaining patient safety. It also supports compliance during inspections and audits.
More Technology Templates