A Change Control Document Sample for Process Modification provides a structured template to record proposed changes, justifications, and impact assessments. It ensures systematic evaluation and approval of process adjustments to maintain compliance and operational efficiency. This document helps organizations track modifications while minimizing risks associated with uncontrolled changes.
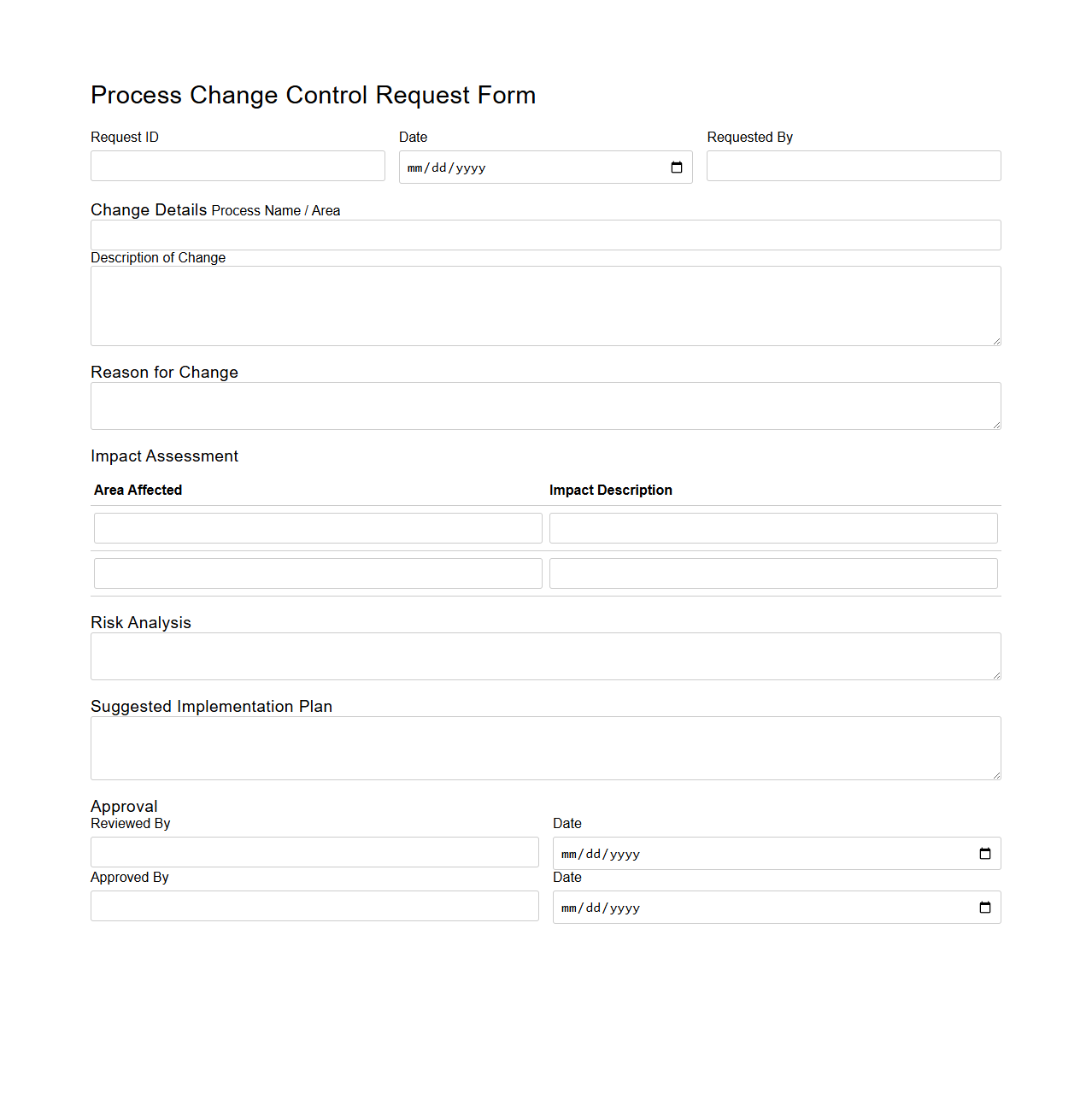
Process Change Control Request Form Template
A
Process Change Control Request Form Template document serves as a standardized tool to formally propose, document, and evaluate modifications within a defined process. It captures essential details such as the description of the change, rationale, potential impact, and approval workflow to ensure controlled and traceable process adjustments. This template is crucial for maintaining consistency, minimizing risks, and supporting compliance in process management systems.
Process Modification Approval Workflow Document
The
Process Modification Approval Workflow Document outlines the formal steps required to review, evaluate, and authorize changes to established processes within an organization. It defines roles, responsibilities, and approval criteria to ensure modifications comply with quality standards and regulatory requirements. This document enhances process control, reduces risks, and ensures consistent implementation of approved changes.
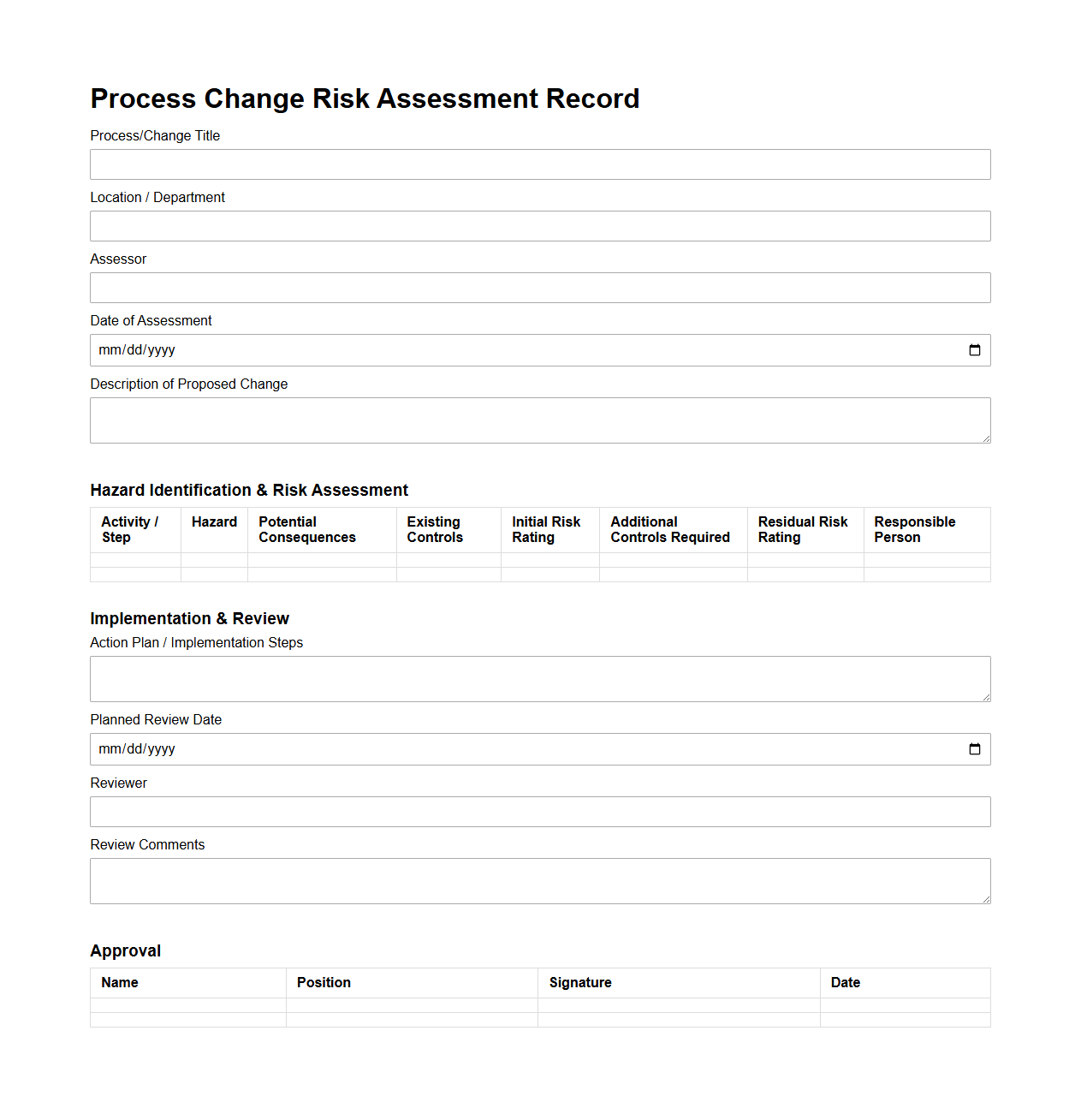
Process Change Risk Assessment Record
The
Process Change Risk Assessment Record document systematically identifies and evaluates potential risks associated with modifications in manufacturing or operational processes to ensure safety, quality, and compliance. It compiles detailed information on the nature of the change, possible hazards, risk levels, and mitigation strategies, facilitating informed decision-making and regulatory adherence. This record serves as a critical reference for continuous improvement and audit purposes in quality management systems.
Process Impact Analysis Report Template
A
Process Impact Analysis Report Template document provides a structured format to evaluate and document the effects of changes within business processes. It helps identify potential risks, benefits, and critical impact areas, ensuring informed decision-making and efficient change management. This template supports consistent reporting and enhances communication among stakeholders by clearly outlining process modifications and their consequences.
Change Implementation Plan Template
A
Change Implementation Plan Template document outlines a structured approach for managing organizational or project changes by detailing key steps, resources, timelines, and responsibilities. It serves as a strategic guide to ensure smooth transition, minimize disruption, and align stakeholders with the change objectives. The template typically includes sections for risk assessment, communication strategies, training plans, and success metrics to monitor progress effectively.
Process Modification Communication Log
The
Process Modification Communication Log document serves as a centralized record tracking all communications related to changes made in a business or manufacturing process. It captures details such as the nature of the modification, stakeholders involved, dates of correspondence, and approval statuses to ensure transparency and accountability. This log is essential for maintaining compliance, facilitating audits, and supporting continuous process improvement initiatives.
Change Verification and Validation Checklist
The
Change Verification and Validation Checklist document ensures systematic evaluation of all modifications in a project or system to confirm they meet specified requirements and function correctly. It guides teams through verifying changes, validating their impact, and identifying potential issues before deployment. This checklist helps maintain quality control, reduce risks, and ensure successful implementation of changes.
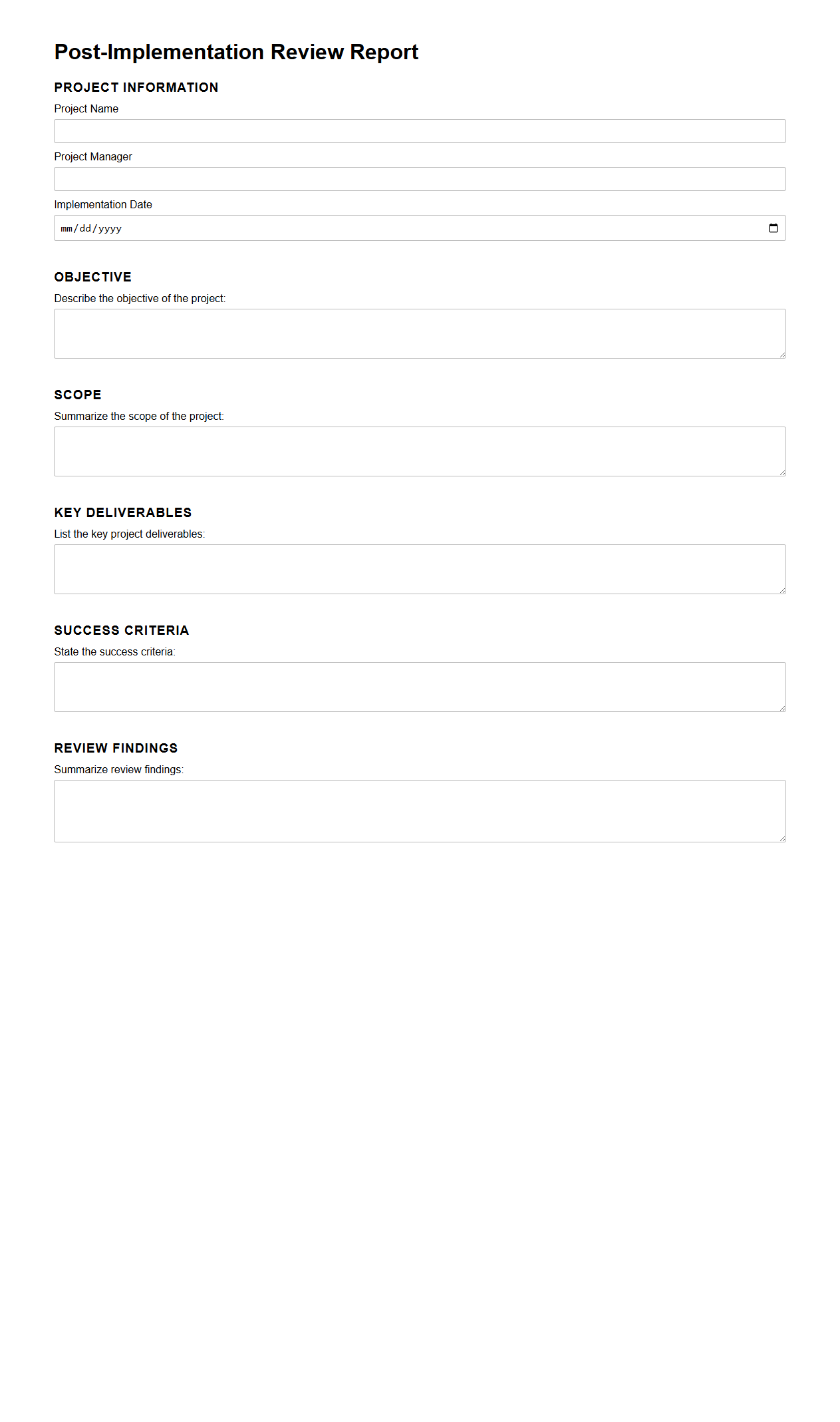
Post-Implementation Review Report Template
A
Post-Implementation Review Report Template document is a structured tool used to evaluate the success and effectiveness of a project after its completion. It systematically captures key metrics, lessons learned, stakeholder feedback, and any deviations from the original plan to inform future projects. This template helps organizations improve project management practices and ensure alignment with business objectives.
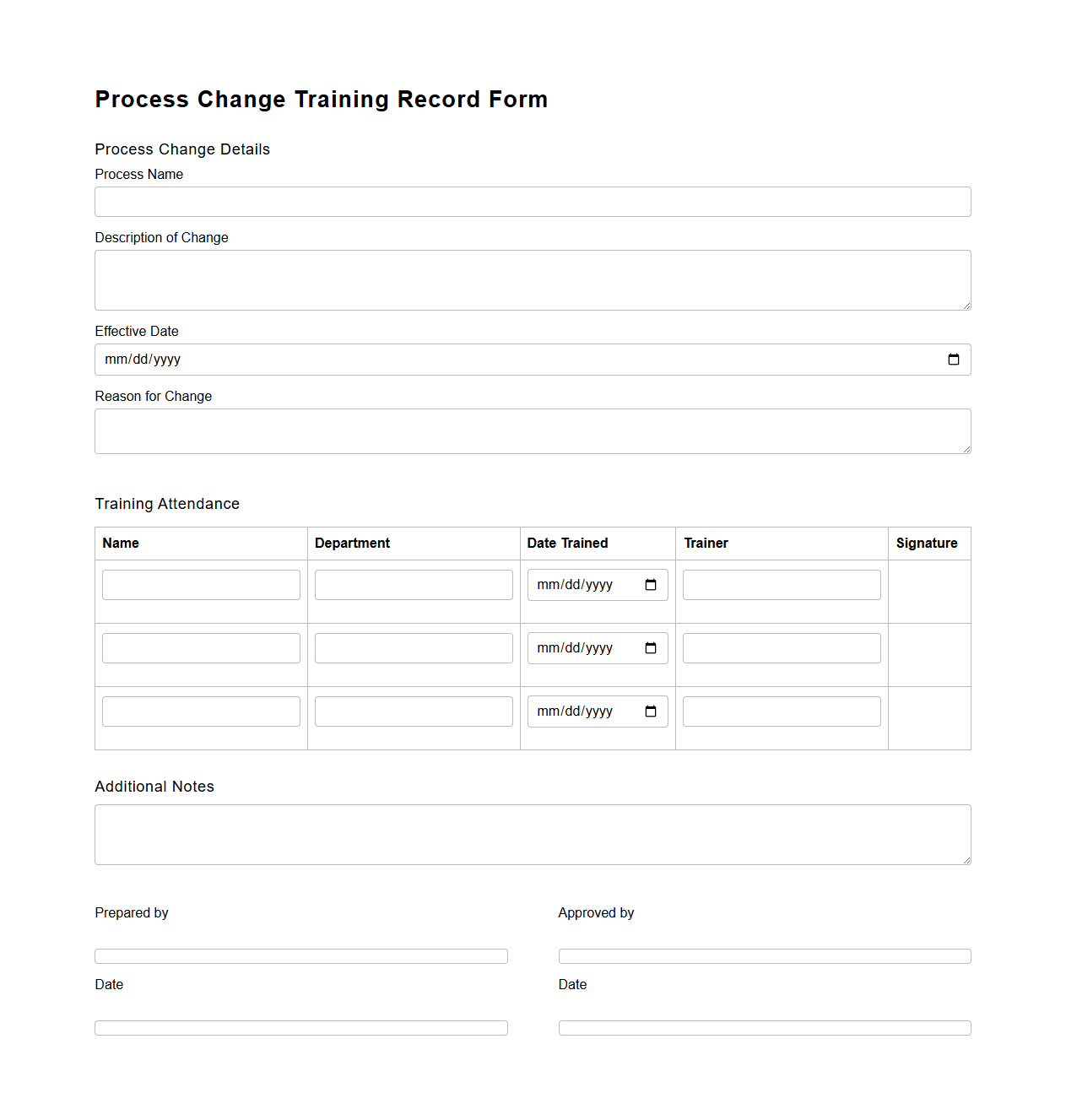
Process Change Training Record Form
The
Process Change Training Record Form document tracks employee training related to updates or modifications in operational procedures. It ensures that all personnel affected by process changes receive proper instruction, promoting compliance and minimizing errors. This form serves as a verified record for audits and quality management systems, demonstrating adherence to updated protocols.
Process Modification Stakeholder Sign-Off Sheet
The
Process Modification Stakeholder Sign-Off Sheet document serves as an official record capturing approval from all relevant stakeholders involved in a proposed change to existing processes. It outlines the specifics of the modification, potential impacts, and ensures alignment and accountability by requiring signatures from decision-makers before implementation. This document is critical for maintaining transparency and securing consensus in process management workflows.
What is the primary objective of the process modification described in the Change Control Document?
The primary objective of the process modification is to enhance the efficiency of current operations by streamlining workflows. This modification aims to reduce errors and improve overall productivity. Ultimately, the change seeks to align the process with updated compliance standards.
Which departments or stakeholders are impacted by the proposed changes in the document?
The proposed changes impact multiple departments, including Quality Assurance, Production, and Supply Chain. Key stakeholders such as department managers and process owners will also be directly involved. Additionally, external vendors might need to adjust their interactions based on the new processes.
What are the key risks and mitigation strategies identified for this process modification?
One key risk involves potential disruptions during the transition period, which could affect production timelines. To mitigate this, a phased implementation plan with continuous monitoring is recommended. Another risk is inadequate training, addressed by comprehensive staff education and support resources.
How will the success of the process change be measured and validated post-implementation?
Success will be measured through key performance indicators (KPIs) such as error rate reduction and turnaround time improvements. Validation will include audits and process reviews to ensure compliance and effectiveness. Employee feedback and system performance data will further support evaluation efforts.
What are the required approvals and documentation steps outlined before change execution?
The change control document requires formal approvals from department heads and the Change Control Board. Documentation steps include detailed change descriptions, risk assessments, and testing plans. All approvals must be recorded before initiating the process modification.
More Manufacturing Templates