A Manufacturing Change Control Document Sample for Manufacturing outlines the procedures and approvals required to manage changes in the production process effectively. It ensures all modifications are documented, evaluated for impact, and implemented consistently to maintain product quality and compliance. This sample serves as a standardized template to streamline change management within manufacturing operations.
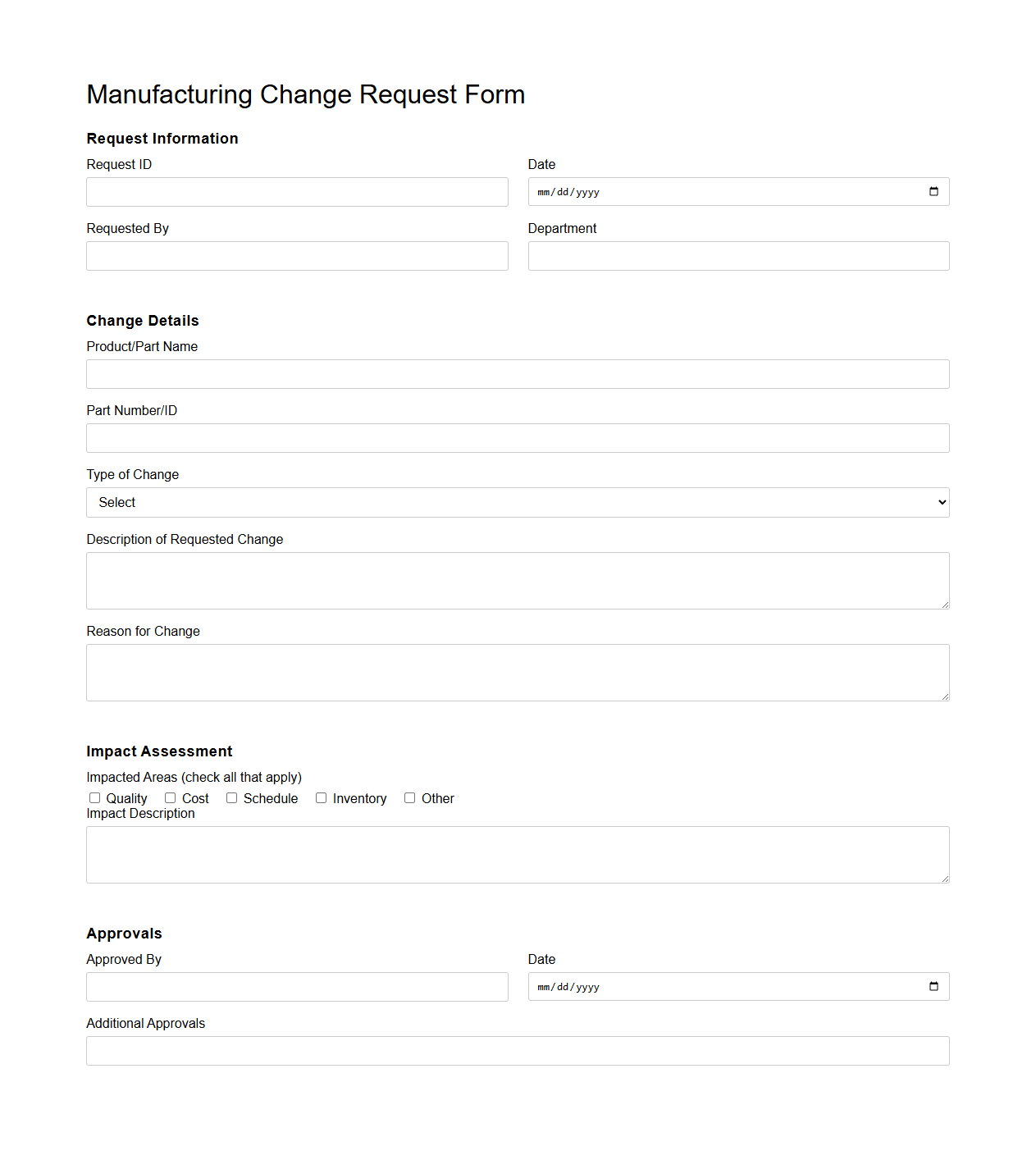
Manufacturing Change Request Form Template
A
Manufacturing Change Request Form Template document streamlines the process of proposing, reviewing, and approving modifications in manufacturing operations. It captures critical information such as the nature of the change, impact analysis, and authorization signatures to ensure transparent communication and accountability. This template enhances compliance with industry standards and reduces risks associated with uncontrolled process alterations.
Engineering Change Order Document Example
An
Engineering Change Order Document example serves as a formal record detailing modifications to a product's design, specifications, or manufacturing process. It outlines the reason for the change, affected components, implementation steps, and approval workflow to ensure accurate communication among engineering, production, and quality teams. This document is essential for maintaining product integrity, tracking revisions, and minimizing errors during the change management process.
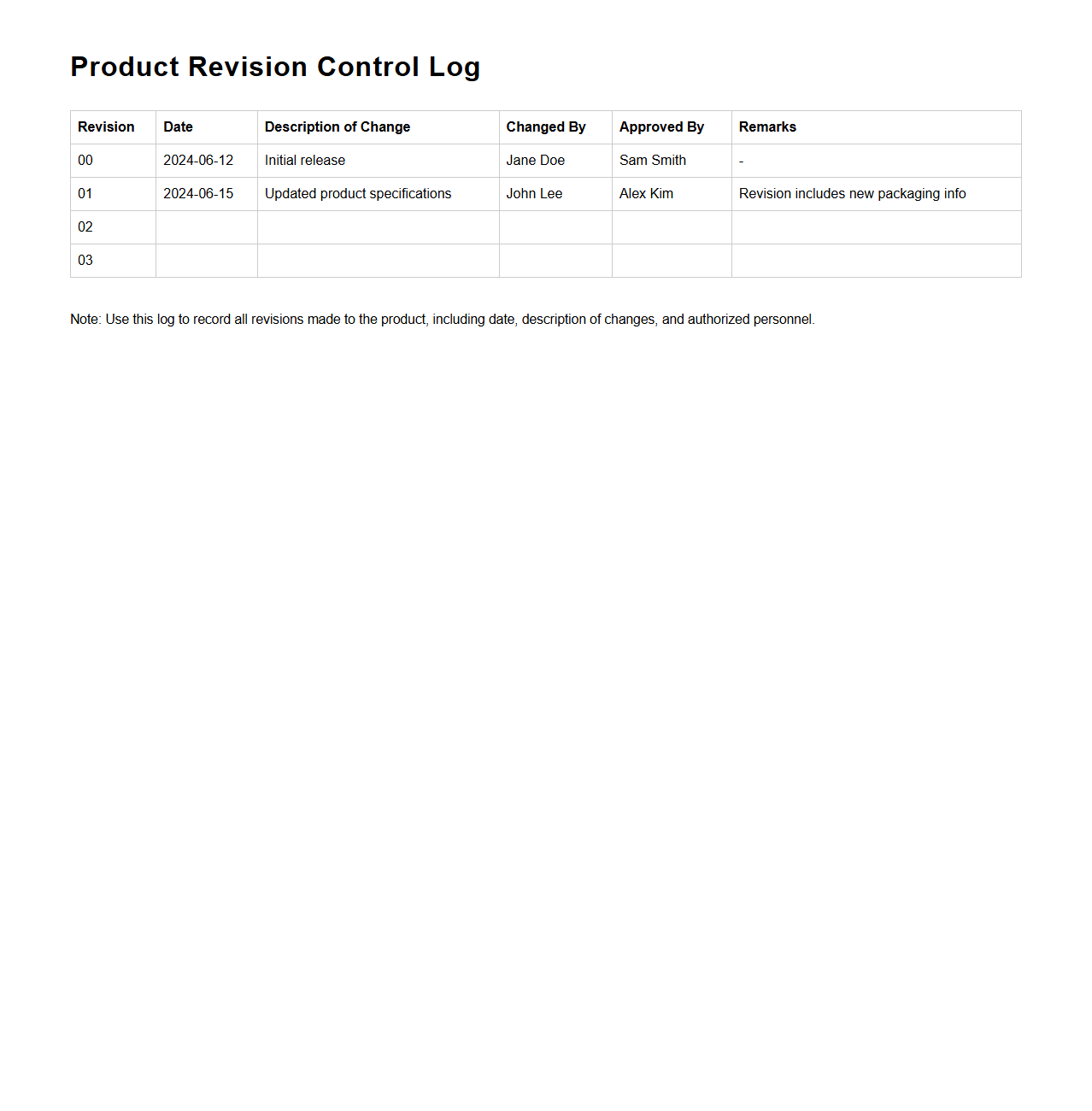
Product Revision Control Log Sample
A
Product Revision Control Log Sample document is a structured record used to track changes made to product designs, specifications, or versions throughout its lifecycle. It details revision numbers, descriptions of changes, dates, and responsible personnel, ensuring clear communication and traceability. This log plays a critical role in quality management and compliance by providing an organized method to monitor product updates systematically.
Design Change Notification Form
A
Design Change Notification Form is a formal document used to communicate proposed modifications to an existing design within a project or product lifecycle. It captures essential details such as the scope of change, reasons, affected components, and approval status, ensuring clear traceability and stakeholder awareness. This form plays a critical role in maintaining quality control and aligning all teams with updated design specifications.
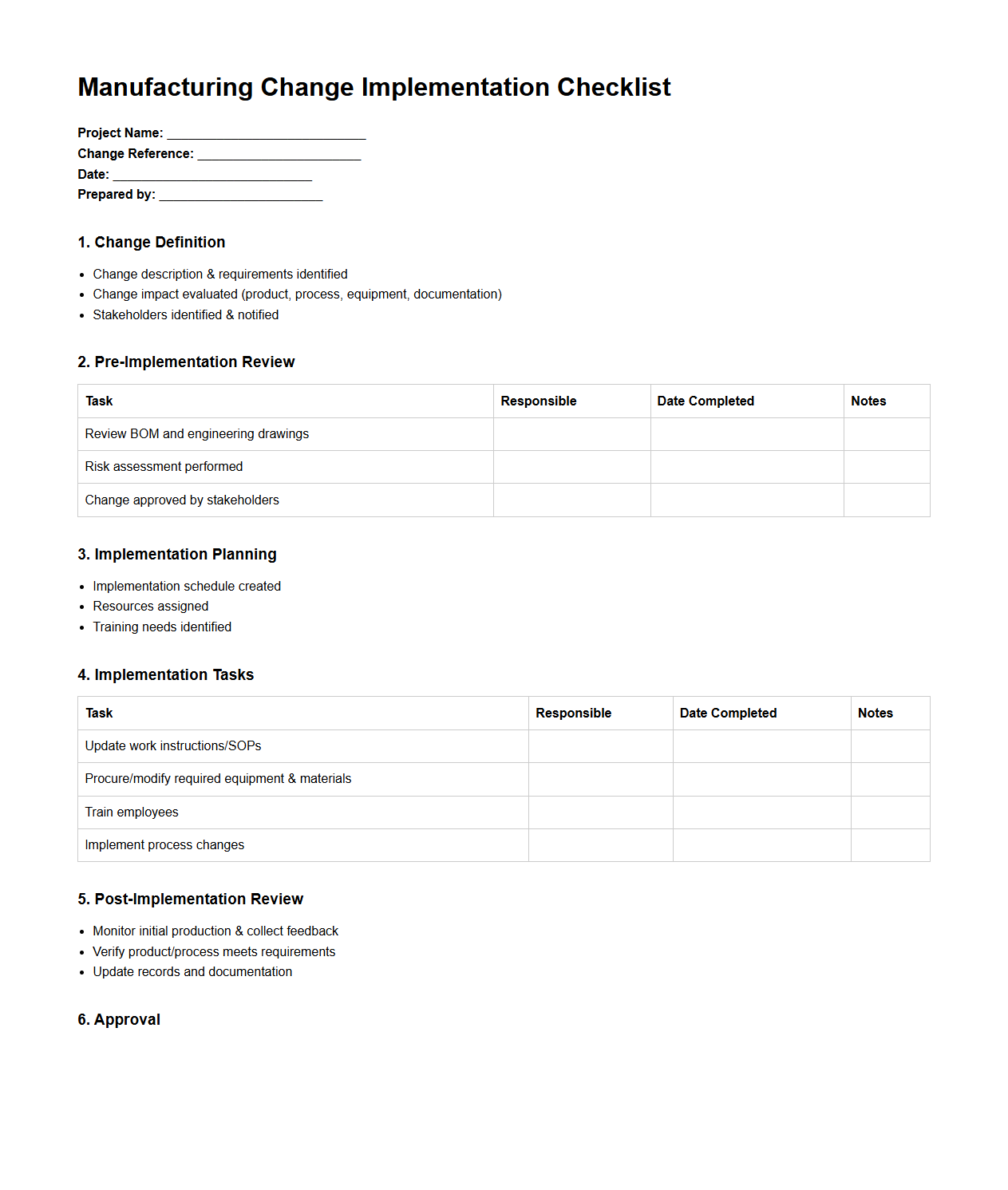
Manufacturing Change Implementation Checklist
A
Manufacturing Change Implementation Checklist document is a structured tool used to ensure all necessary steps are followed during the introduction of changes in manufacturing processes. It helps track compliance with quality standards, risk assessments, validation procedures, and communication protocols to maintain production efficiency and product integrity. This checklist minimizes errors, reduces downtime, and supports regulatory compliance in manufacturing environments.
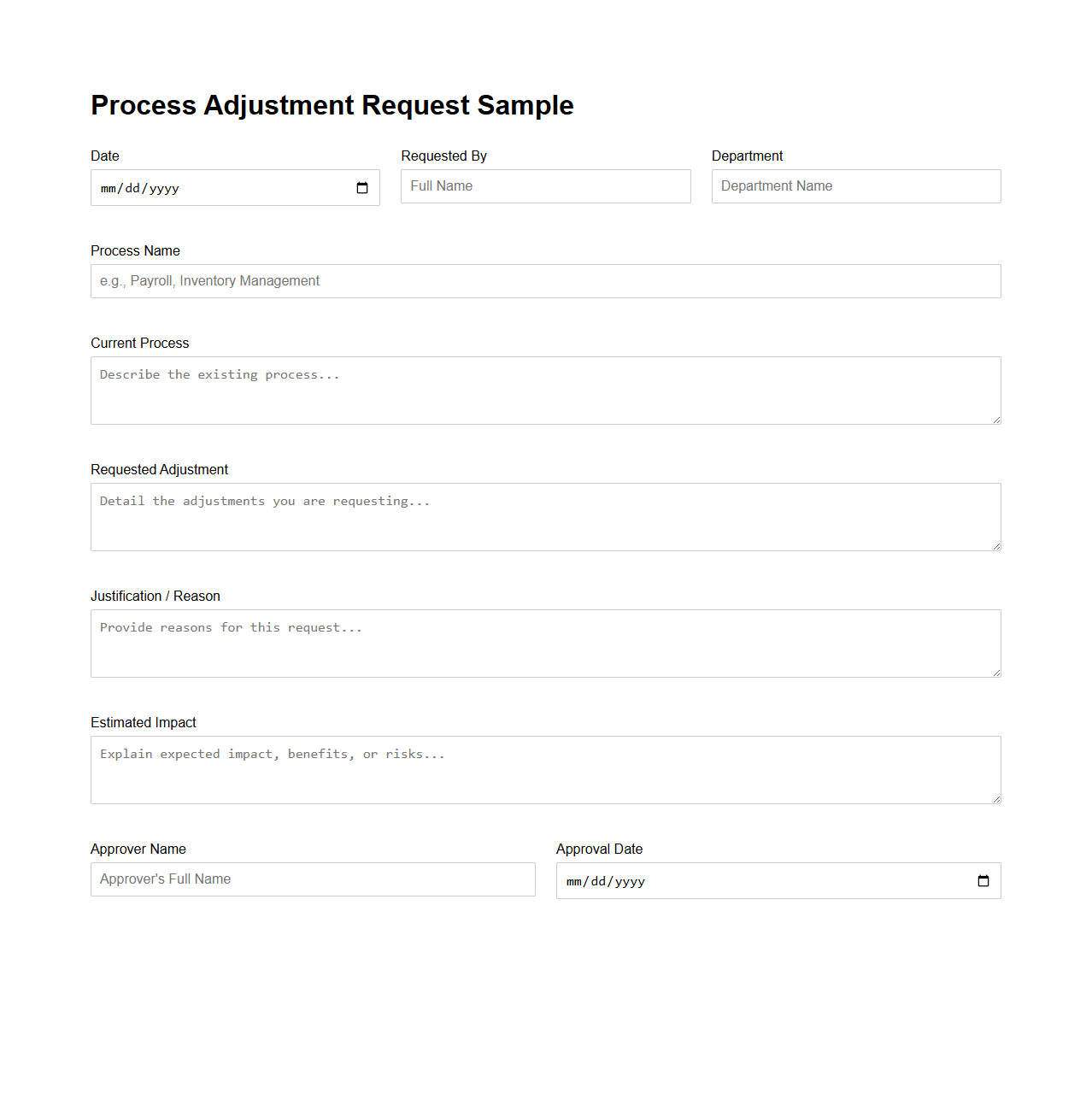
Process Adjustment Request Sample
A
Process Adjustment Request Sample document serves as a formal template used to propose modifications in existing operational procedures within an organization. It outlines the current process, the desired changes, the rationale behind the adjustment, and expected outcomes to ensure clarity and facilitate approval. This document helps streamline workflow improvements, enhance efficiency, and maintain compliance with company standards.
Change Control Approval Routing Sheet
The
Change Control Approval Routing Sheet document is a critical tool used in project management and quality assurance to track and authorize changes within a process or product. It ensures all proposed modifications are reviewed, evaluated, and approved by designated stakeholders, maintaining compliance with organizational standards. This document helps streamline communication, reduces errors, and provides a clear audit trail for regulatory and operational purposes.
Manufacturing Specification Update Form
The
Manufacturing Specification Update Form document serves as a formal record to capture any changes or updates to manufacturing specifications, ensuring accuracy and consistency in production processes. It outlines detailed modifications to parameters such as materials, dimensions, tolerances, or assembly instructions, which are essential for quality control and compliance with industry standards. Maintaining this document helps streamline communication between engineering, production, and quality assurance teams, reducing errors and minimizing production delays.
Bill of Materials Change Record
A
Bill of Materials Change Record document tracks modifications made to the original bill of materials (BOM) for a product, ensuring proper version control and accurate production details. It includes information such as change description, date, reason, approval status, and the affected component list. Maintaining this record improves communication across manufacturing, engineering, and procurement teams while preventing errors and ensuring compliance with industry standards.
Process Validation Change Summary
The
Process Validation Change Summary document provides a detailed record of any modifications made to validated processes within manufacturing or quality systems. It ensures that all changes are systematically reviewed, approved, and documented to maintain compliance with regulatory standards such as FDA and ISO. This summary helps track the impact of changes on process performance, product quality, and overall system integrity.
What is the primary purpose of a Manufacturing Change Control Document in the production process?
The primary purpose of a Manufacturing Change Control Document is to manage and control modifications in the production process systematically. It ensures that all changes are evaluated for potential impacts on product quality and safety before implementation. This document helps maintain consistency and traceability throughout the manufacturing lifecycle.
Which key stakeholders are required to review and approve manufacturing changes as outlined in the document?
The key stakeholders typically include Quality Assurance, Production Management, Regulatory Affairs, and Engineering teams. These groups collaborate to assess the risks and benefits of proposed changes. Their approval is crucial to ensure all aspects of the change align with organizational and regulatory standards.
How are proposed changes documented and tracked within the Manufacturing Change Control system?
Proposed changes are documented using standardized forms or electronic systems that capture detailed descriptions, reasons, and potential impacts. Each change is assigned a unique identifier for effective tracking through various review stages. This process promotes transparency and accountability in managing manufacturing modifications.
What criteria determine whether a manufacturing change requires validation before implementation?
A manufacturing change requires validation if it impacts critical process parameters, product quality attributes, or regulatory compliance. Changes affecting safety, efficacy, or performance also necessitate thorough validation. The decision is based on a risk assessment performed during the review process.
How does the document ensure compliance with regulatory and quality system requirements?
The document incorporates regulatory and quality system requirements by referencing applicable standards and guidelines. It mandates documented approvals, risk assessments, and validation activities to meet compliance criteria. This structured approach helps organizations maintain audit readiness and product integrity.
More Manufacturing Templates