A Nonconformance Report Document Sample for Manufacturing outlines the process of identifying, documenting, and addressing deviations from production standards. It serves as a critical tool to track defects, analyze root causes, and implement corrective actions to improve product quality. Proper use of this document enhances compliance with industry regulations and boosts overall manufacturing efficiency.
Manufacturing Nonconformance Incident Report Template
The
Manufacturing Nonconformance Incident Report Template document is a standardized form used to record and analyze deviations from manufacturing standards or specifications. It helps in documenting the nature of the nonconformance, its root causes, and corrective actions taken to prevent recurrence. This template supports quality control processes by ensuring consistent incident reporting and effective resolution tracking.
Product Deviation Report Format for Quality Control
The
Product Deviation Report Format for Quality Control is a standardized document used to record and analyze deviations from specified product standards during manufacturing or inspection. It captures essential details such as the nature of the deviation, root cause analysis, corrective actions, and approvals to ensure compliance with quality requirements. This format enables systematic tracking, improves accountability, and supports continuous improvement in product quality management.
Nonconformance Root Cause Analysis Form for Manufacturing
The
Nonconformance Root Cause Analysis Form for manufacturing is a critical document used to systematically identify and document the underlying causes of product or process nonconformities. It facilitates effective corrective actions by capturing detailed information on defects, deviations, and process failures, ensuring continuous quality improvement. This form supports compliance with industry standards such as ISO 9001 and enhances traceability throughout the production cycle.
Manufacturing Process Nonconformance Log Example
A
Manufacturing Process Nonconformance Log Example document serves as a critical tool to record and track deviations from established production standards and specifications. It details the nature of nonconformances, including the affected product, date and time of occurrence, root cause analysis, and corrective actions taken to prevent recurrence. This document facilitates continuous quality improvement by enabling manufacturers to systematically identify, analyze, and resolve process inefficiencies and defects.
Corrective Action Report for Production Nonconformance
A
Corrective Action Report for Production Nonconformance is a formal document used to identify, analyze, and address deviations from manufacturing standards or specifications. It records the root cause of the nonconformance, outlines corrective measures to prevent recurrence, and tracks the implementation and effectiveness of these actions. This report is essential for maintaining quality control, ensuring compliance with industry regulations, and improving overall production processes.
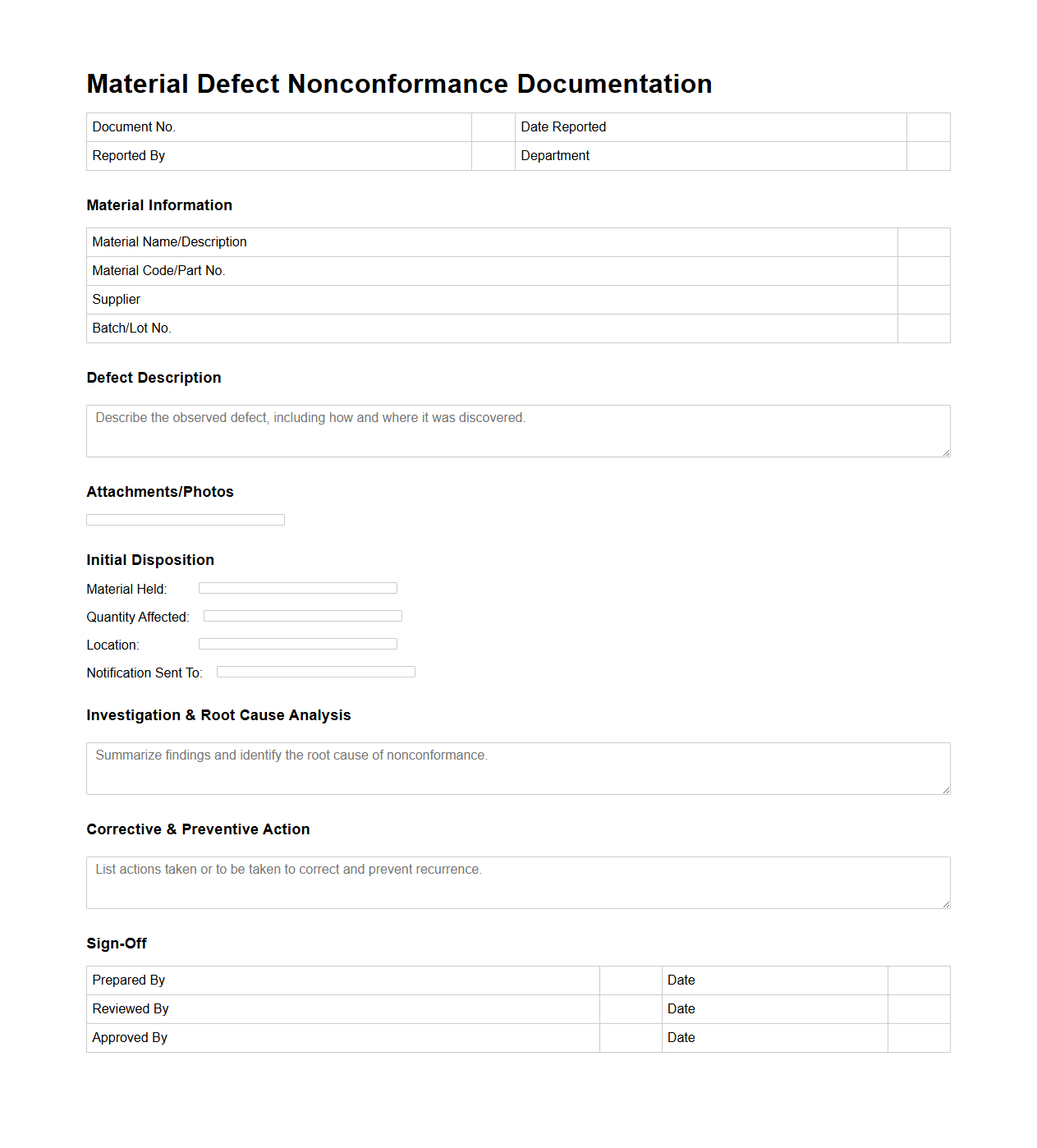
Material Defect Nonconformance Documentation Sample
A
Material Defect Nonconformance Documentation Sample document is used to record and report discrepancies or defects in materials that do not meet specified standards or quality requirements. This documentation typically includes details such as the nature of the defect, identification numbers, inspection results, and corrective actions taken. It serves as an essential tool for quality control teams to monitor, analyze, and resolve material-related issues efficiently and maintain compliance with industry standards.
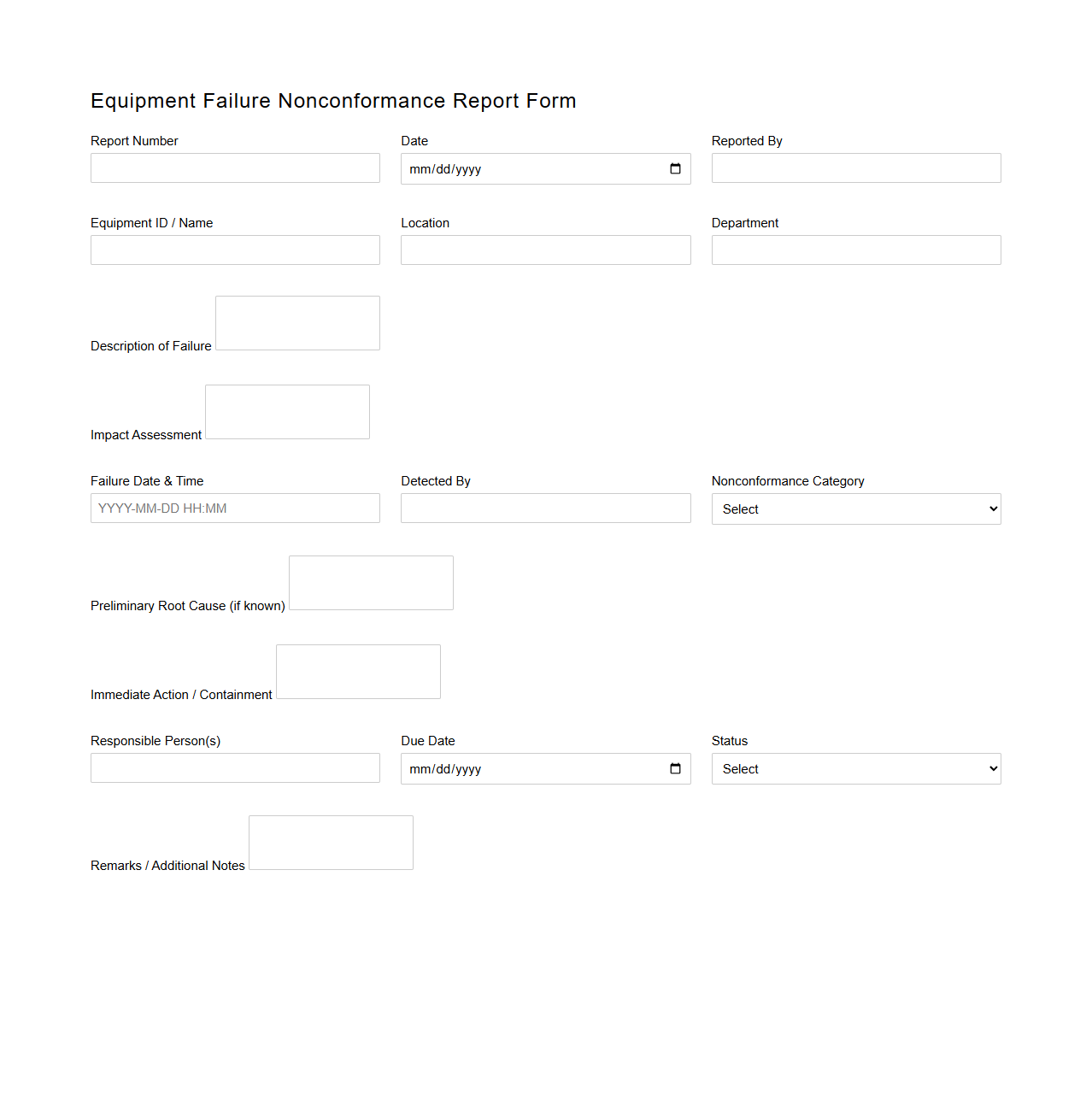
Equipment Failure Nonconformance Report Form
The
Equipment Failure Nonconformance Report Form document is used to systematically record and analyze instances of equipment malfunction or breakdown, ensuring accurate tracking of defects. It captures critical data such as failure descriptions, root causes, and corrective actions taken, facilitating effective maintenance and quality control. This form supports compliance with industry standards by providing traceable documentation to prevent recurrence and improve equipment reliability.
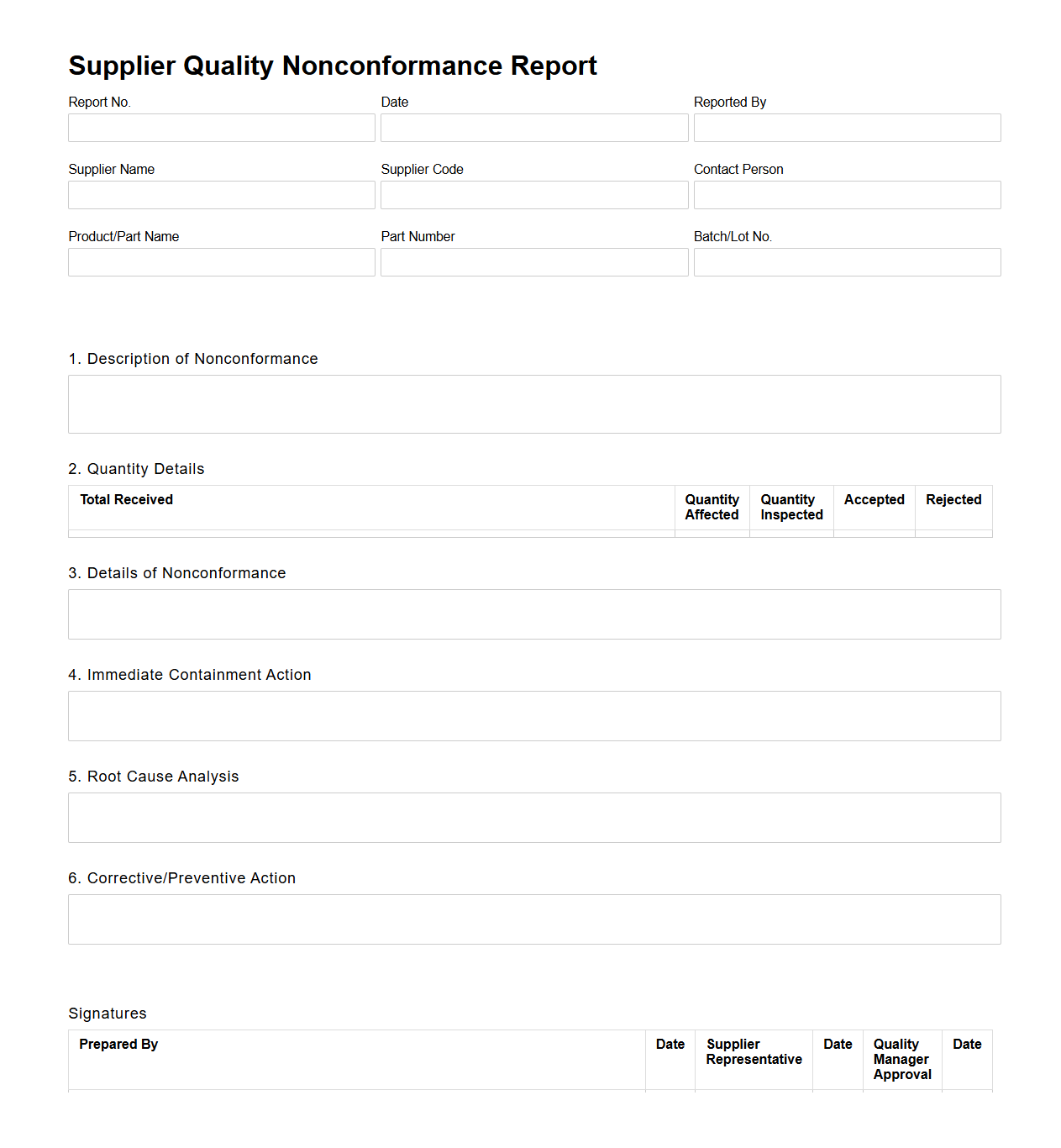
Supplier Quality Nonconformance Report for Manufacturing
A
Supplier Quality Nonconformance Report for manufacturing is a formal document used to identify and record instances where supplied materials or components do not meet specified quality standards or contractual requirements. This report details the nature of the nonconformance, its source, and the potential impact on production, enabling manufacturers to initiate corrective actions and prevent recurrence. It serves as a critical tool in maintaining supply chain integrity and ensuring product quality compliance.
Assembly Line Nonconformance Tracking Sheet
The
Assembly Line Nonconformance Tracking Sheet document serves as a critical tool for monitoring and recording defects or deviations encountered during the manufacturing process on an assembly line. It systematically captures detailed information about nonconformances, including description, location, time, and responsible personnel, enabling effective root cause analysis and corrective action planning. This tracking sheet supports quality control efforts by ensuring that issues are promptly identified, documented, and resolved to maintain production standards and minimize rework or scrap rates.
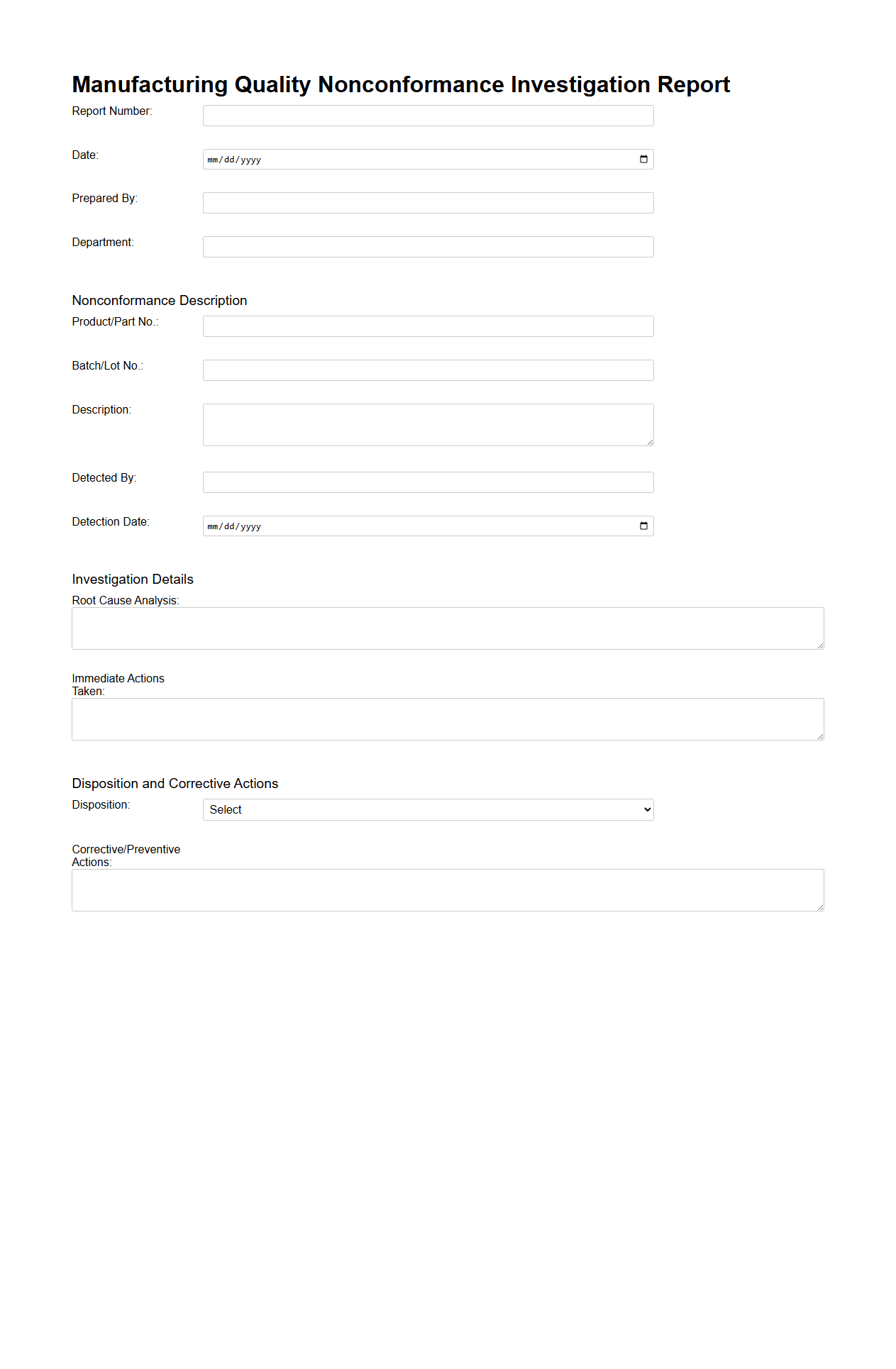
Manufacturing Quality Nonconformance Investigation Report
A
Manufacturing Quality Nonconformance Investigation Report document details the identification, analysis, and resolution of deviations from specified quality standards during the manufacturing process. It includes information on the root cause, corrective actions, and preventive measures to ensure product compliance and maintain production integrity. This report is essential for continuous improvement and regulatory compliance in manufacturing operations.
What specific quality standards or specifications does the Nonconformance Report document reference for evaluating product defects?
The Nonconformance Report (NCR) references international quality standards such as ISO 9001 and industry-specific guidelines to evaluate product defects systematically. It includes detailed specifications related to product dimensions, material properties, and performance criteria that must be met. These standards ensure consistent assessment and maintain product integrity.
Which key sections in the report capture root cause analysis and corrective actions for manufacturing nonconformance?
The NCR includes dedicated sections labeled Root Cause Analysis and Corrective Actions & Preventive Measures (CAPA). The Root Cause Analysis section identifies underlying reasons for defects, using tools like fishbone diagrams or 5 Whys. The Corrective Actions section outlines steps to eliminate causes and prevent recurrence, ensuring continuous quality improvement.
How does the document define the roles and responsibilities for reporting, reviewing, and closing nonconformance cases?
The NCR document clearly assigns roles such as Quality Control Inspectors for identifying and reporting issues, while Quality Managers review findings and approve corrective steps. Production staff are responsible for implementing corrective actions, and the Quality Assurance team ensures proper closure. This delineation enforces accountability throughout the nonconformance resolution process.
What traceability measures are included in the report to link nonconformance incidents to specific batches, lots, or production dates?
The report incorporates traceability via batch numbers, lot codes, and production dates recorded meticulously in the NCR form. This information connects each nonconformance event to its origin, facilitating effective investigation and containment. Traceability also supports recall procedures if widespread defects emerge.
How does the Nonconformance Report document ensure compliance with regulatory and customer requirements during the review process?
The NCR workflow integrates checkpoints to verify adherence to regulatory standards and customer-specific requirements before case closure. It requires documented evidence that all corrective activities meet compliance obligations. Auditing trails maintain transparency, ensuring that the review process satisfies both legal and contractual expectations.
More Manufacturing Templates