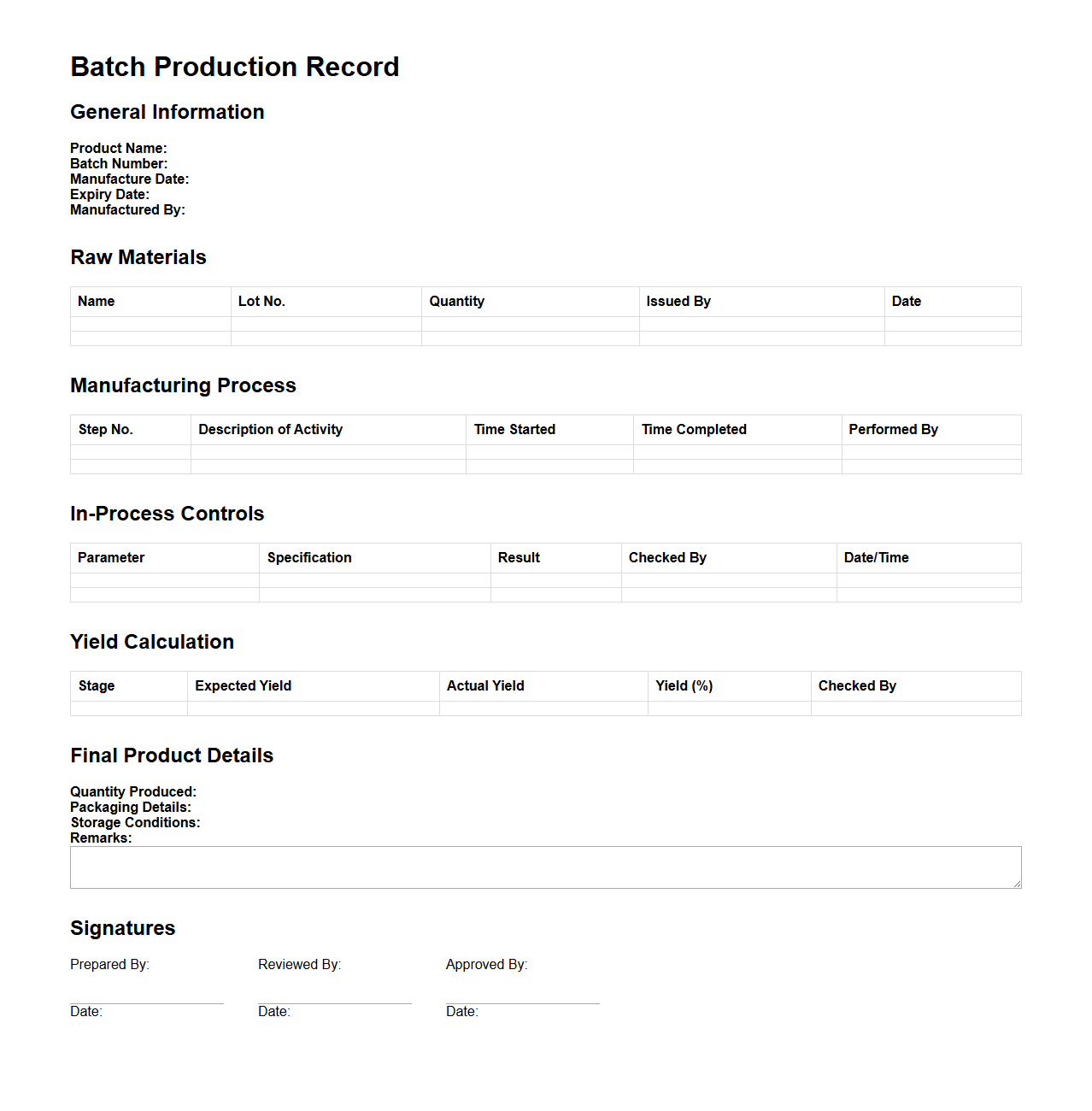
Batch Production Record Template for Pharmaceutical Manufacturing
A
Batch Production Record Template for pharmaceutical manufacturing is a standardized document that details every step involved in the production of a specific batch of a drug product. It serves as a comprehensive guide and record, ensuring consistency, compliance with regulatory standards, and traceability throughout the manufacturing process. This template includes critical information such as raw material specifications, equipment used, processing instructions, in-process controls, and final product testing results.
Batch Process Sheet Example for Food Industry
A
Batch Process Sheet Example for the food industry is a detailed document outlining the step-by-step procedures, ingredient quantities, equipment settings, and safety checks required to produce a specific food product batch consistently and efficiently. It serves as a critical guide for production teams, ensuring quality control, traceability, and compliance with regulatory standards. This document also helps in monitoring production performance and identifying areas for process improvement.
Batch Manufacturing Order Form for Chemical Plants
A
Batch Manufacturing Order Form for chemical plants is a critical document used to outline precise instructions for producing a specific batch of chemical products, ensuring consistency and quality control. It includes detailed information such as raw material specifications, quantities, process parameters, safety guidelines, and equipment requirements. This form serves as a vital communication tool between production teams and quality assurance to maintain compliance with industry standards and regulatory requirements.
Batch Production Tracking Log for Electronics Assembly
The
Batch Production Tracking Log for Electronics Assembly is a critical document used to monitor and record each stage of the manufacturing process for electronic components and devices. It ensures traceability of batches, captures detailed information on materials, assembly dates, equipment used, and quality control results. Maintaining this log helps in identifying defects, managing inventory, and complying with industry standards such as IPC and ISO 9001.
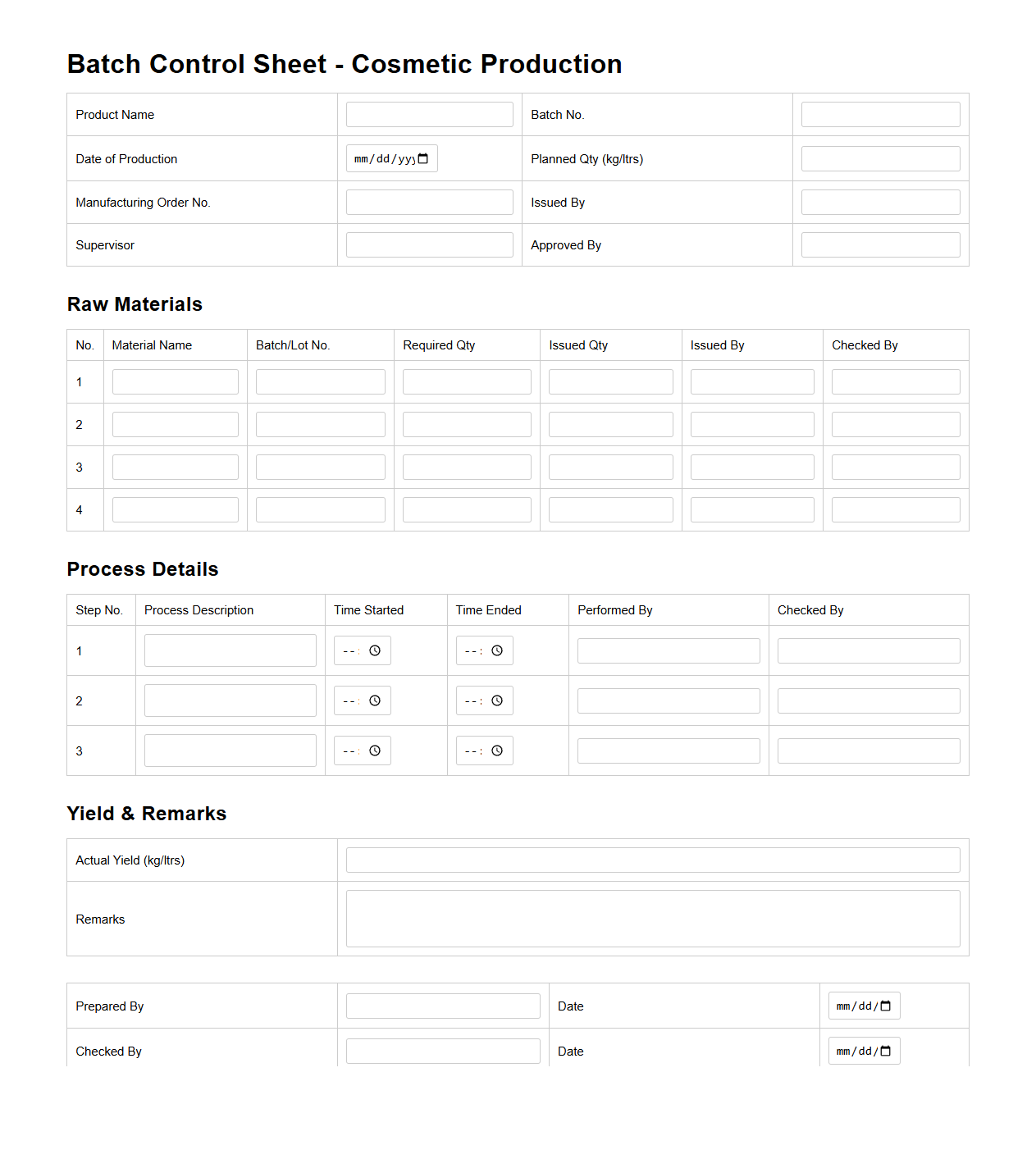
Batch Control Sheet for Cosmetic Production
A
Batch Control Sheet for cosmetic production is a critical document used to record detailed information about each batch manufactured, ensuring traceability and quality compliance. It typically includes data such as raw material quantities, processing parameters, equipment used, and operator details to maintain consistency and facilitate quality assurance audits. This sheet serves as a reference for regulatory bodies and helps in identifying deviations or issues during production.
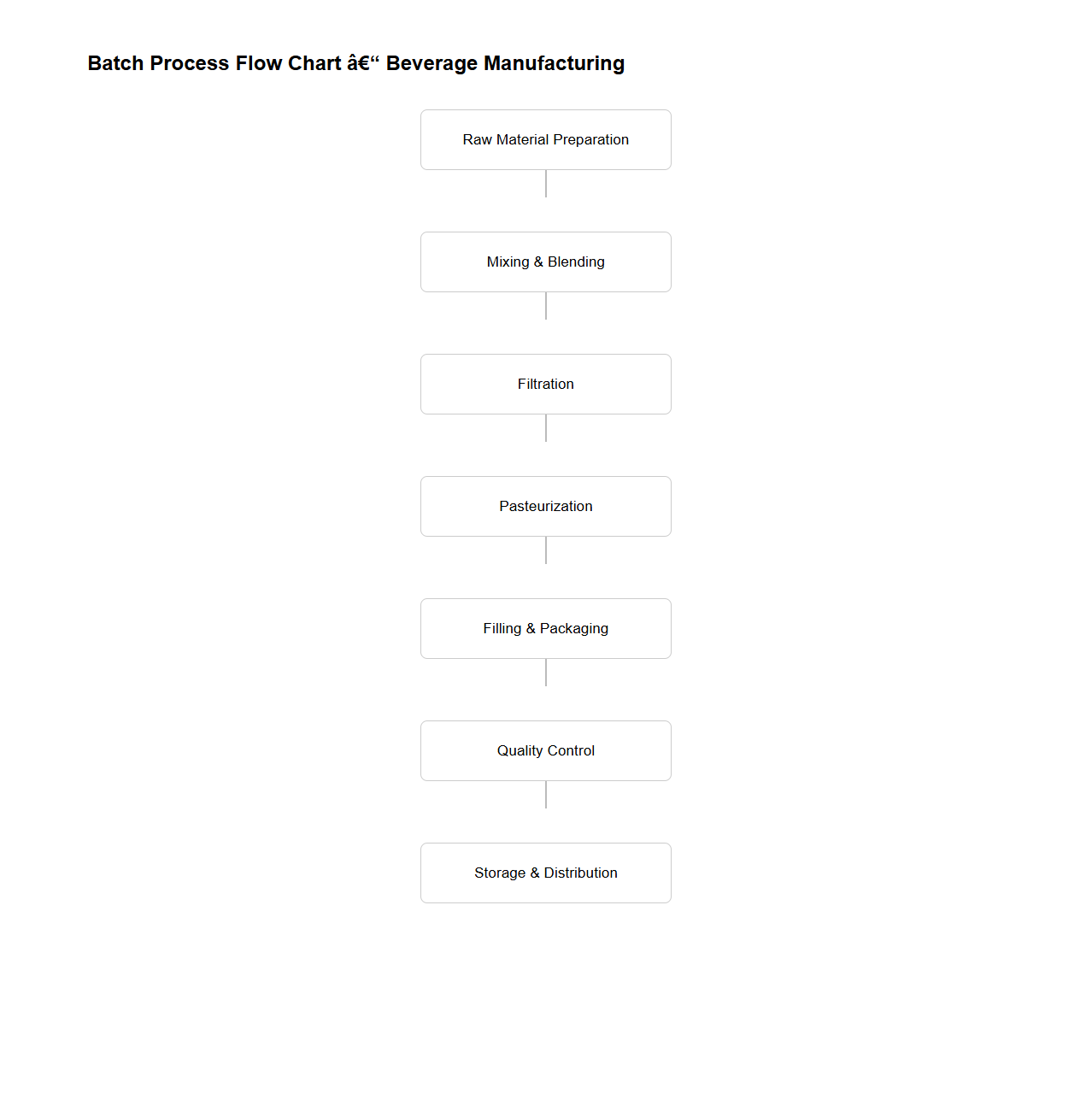
Batch Process Flow Chart for Beverage Manufacturing
A
Batch Process Flow Chart for Beverage Manufacturing document visually represents the sequential steps involved in producing beverages in batches, ensuring standardized quality and efficiency. It details key stages such as raw material preparation, mixing, pasteurization, filling, and packaging, highlighting critical control points and process parameters. This flow chart aids in optimizing production workflows, maintaining regulatory compliance, and facilitating quality assurance in beverage manufacturing operations.
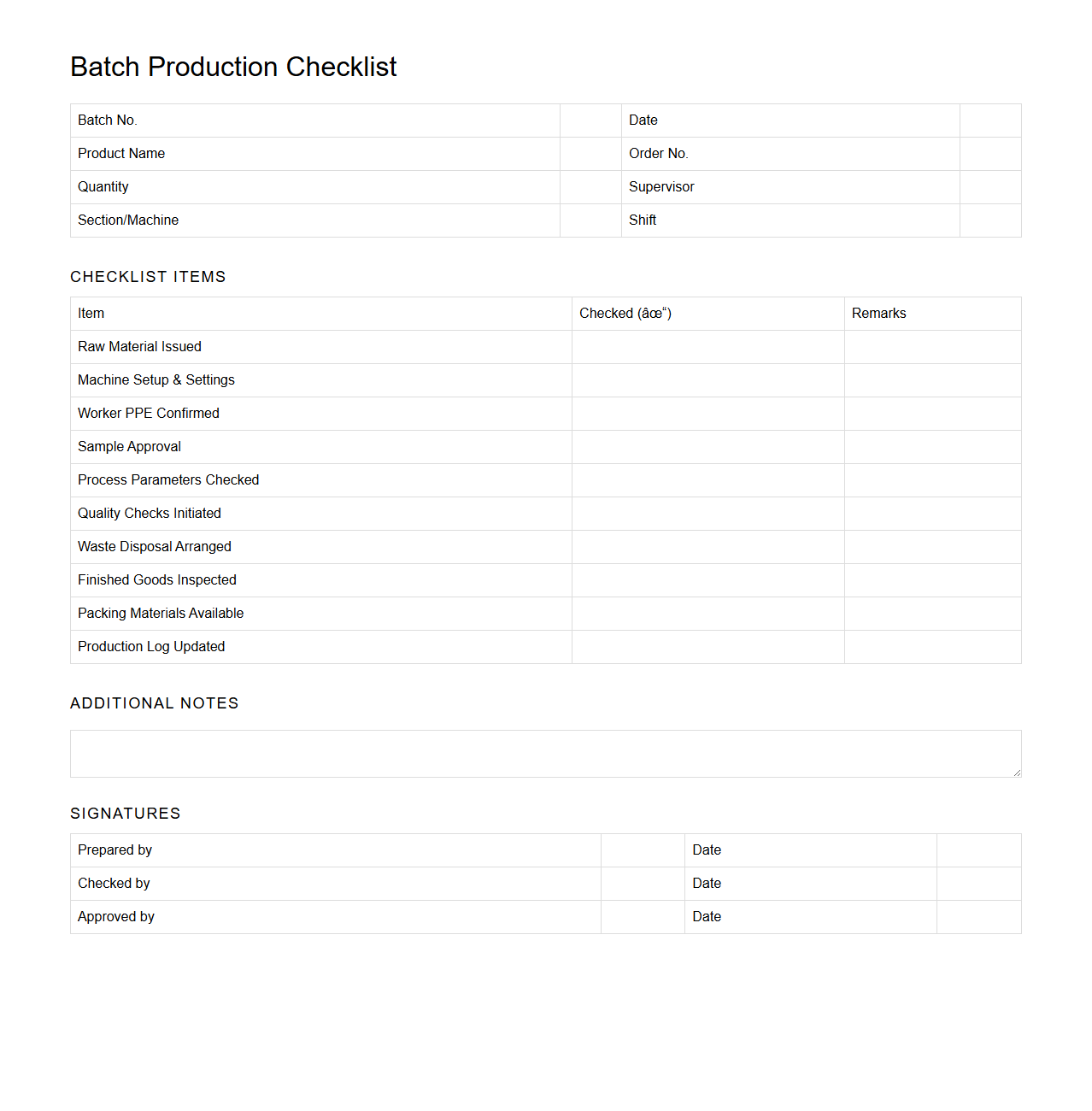
Batch Production Checklist for Textile Industry
The
Batch Production Checklist for the textile industry is a detailed document used to ensure all production steps are completed accurately and efficiently. It outlines key tasks such as raw material verification, machine settings, quality control measures, and safety protocols to maintain consistent product quality. This checklist helps reduce errors, streamline workflow, and improve overall operational efficiency in textile manufacturing.
Batch Manufacturing Report Format for Automotive Components
The
Batch Manufacturing Report Format for Automotive Components document is a structured template used to record detailed production information of automotive parts during a specific manufacturing batch. It includes critical data such as batch number, production date, machine settings, material lot numbers, quality control results, and operator details to ensure traceability and compliance with industry standards. This report facilitates efficient tracking of manufacturing processes, aids in quality assurance, and supports regulatory audits within the automotive sector.
Batch Process Documentation for Paint Manufacturing
Batch Process Documentation for Paint Manufacturing is a detailed record that captures every step involved in producing a specific batch of paint, including raw material quantities, mixing times, temperatures, and quality control checks. This document ensures consistency, traceability, and compliance with industry standards by providing precise instructions and data for each batch. Maintaining accurate
Batch Process Documentation is crucial for optimizing production efficiency and meeting regulatory requirements in the paint manufacturing industry.
Batch Production Summary Sheet for Plastics Fabrication
The
Batch Production Summary Sheet for Plastics Fabrication is a detailed document that records the entire production process of plastic components in a batch. It includes critical data such as material types, quantities used, machine settings, production times, and quality control results to ensure consistent product quality and traceability. This sheet is essential for maintaining production efficiency, managing inventory, and meeting regulatory compliance in plastics manufacturing.
What key information must be included in a batch production document for accurate traceability?
A batch production document must contain batch numbers, production dates, and detailed raw material sources to ensure accurate traceability. It should also record the equipment used, production parameters, and operator details. This comprehensive information allows precise tracking of each batch throughout the manufacturing lifecycle.
How does the document ensure compliance with quality control standards throughout the batch process?
The document includes quality checkpoints and test results at various stages of production to maintain compliance with quality control standards. It mandates adherence to preset specifications and records any deviations or corrective actions taken. This systematic documentation guarantees consistent product quality and regulatory compliance.
What procedures are outlined for handling deviations or non-conformities during batch manufacturing?
The batch production document specifies a deviation reporting system that requires immediate documentation of any non-conformities. It outlines a corrective action plan, including root cause analysis and approval steps before resuming production. This ensures all issues are addressed promptly to maintain product integrity.
How is material usage and inventory tracked and recorded in the batch production document?
Material usage is tracked by recording the quantity of each raw material used per batch along with lot numbers and supplier information. Inventory levels are updated in real-time to reflect consumption and replenish stocks as necessary. This detailed tracking enhances inventory control and prevents material shortages or overuse.
What approval and sign-off steps are required before, during, and after batch production according to the document?
The document requires pre-production approval of materials and processes, continuous monitoring approvals during production, and final sign-off by quality assurance upon batch completion. Each stage must be signed by authorized personnel to validate compliance and readiness to proceed. This multi-level approval system ensures accountability and verifies batch quality.