Sterile Manufacturing Production Batch Record Template
The
Sterile Manufacturing Production Batch Record Template document is a critical tool used to ensure consistency, compliance, and quality control in aseptic pharmaceutical manufacturing processes. It provides a detailed, standardized framework for recording all manufacturing steps, materials, equipment, and environmental conditions involved in the production of sterile products. This template supports regulatory adherence by capturing essential data for traceability, process validation, and batch release decisions.
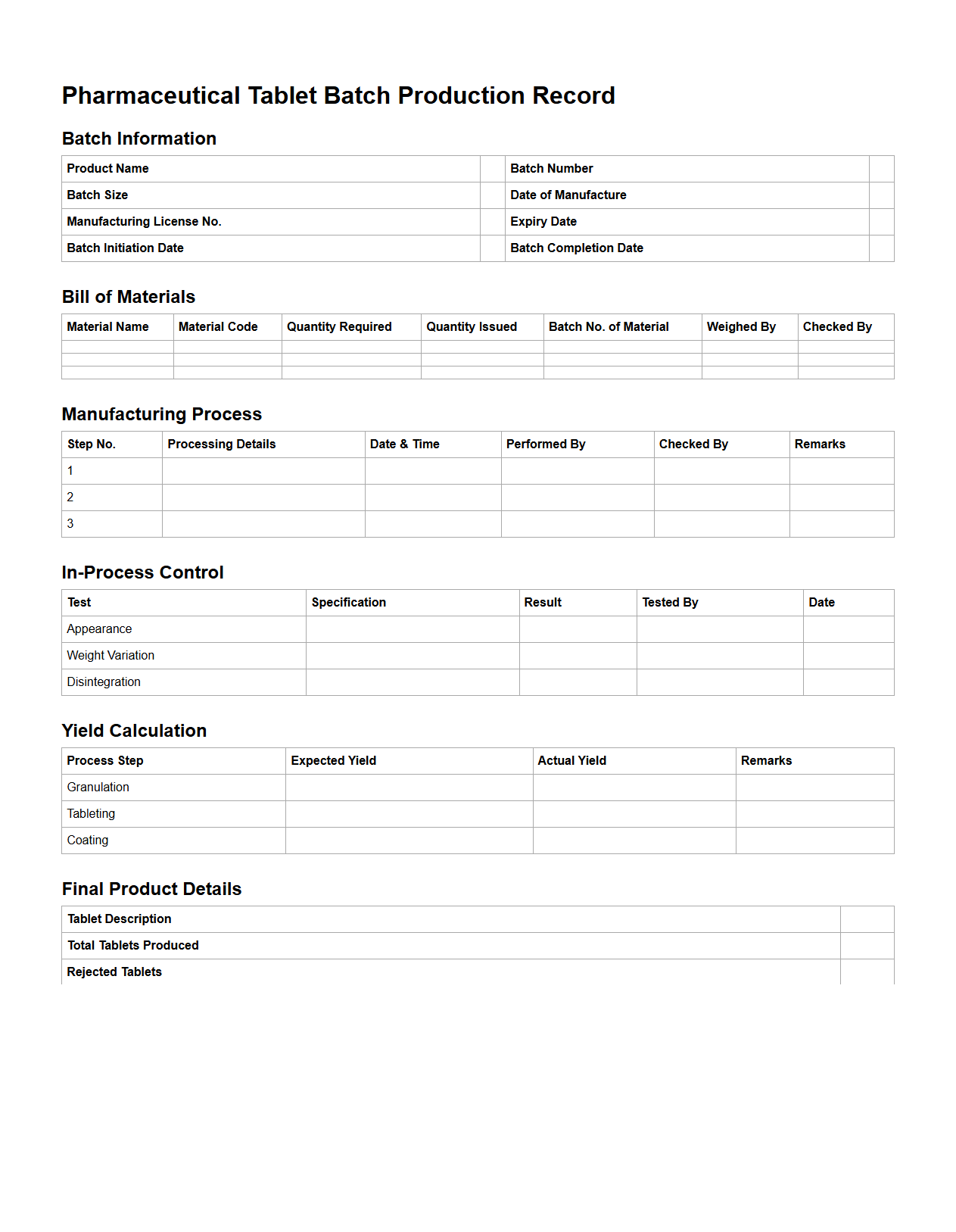
Pharmaceutical Tablet Batch Production Record Example
A
Pharmaceutical Tablet Batch Production Record (BPR) Example document serves as a detailed blueprint for manufacturing a specific batch of tablets, ensuring consistency, quality, and compliance with regulatory standards. It includes essential information such as raw material specifications, equipment used, in-process controls, production steps, and quality checks performed throughout the tablet manufacturing process. This document is crucial for traceability, facilitating batch-to-batch uniformity and enabling investigation in case of deviations or product recalls.
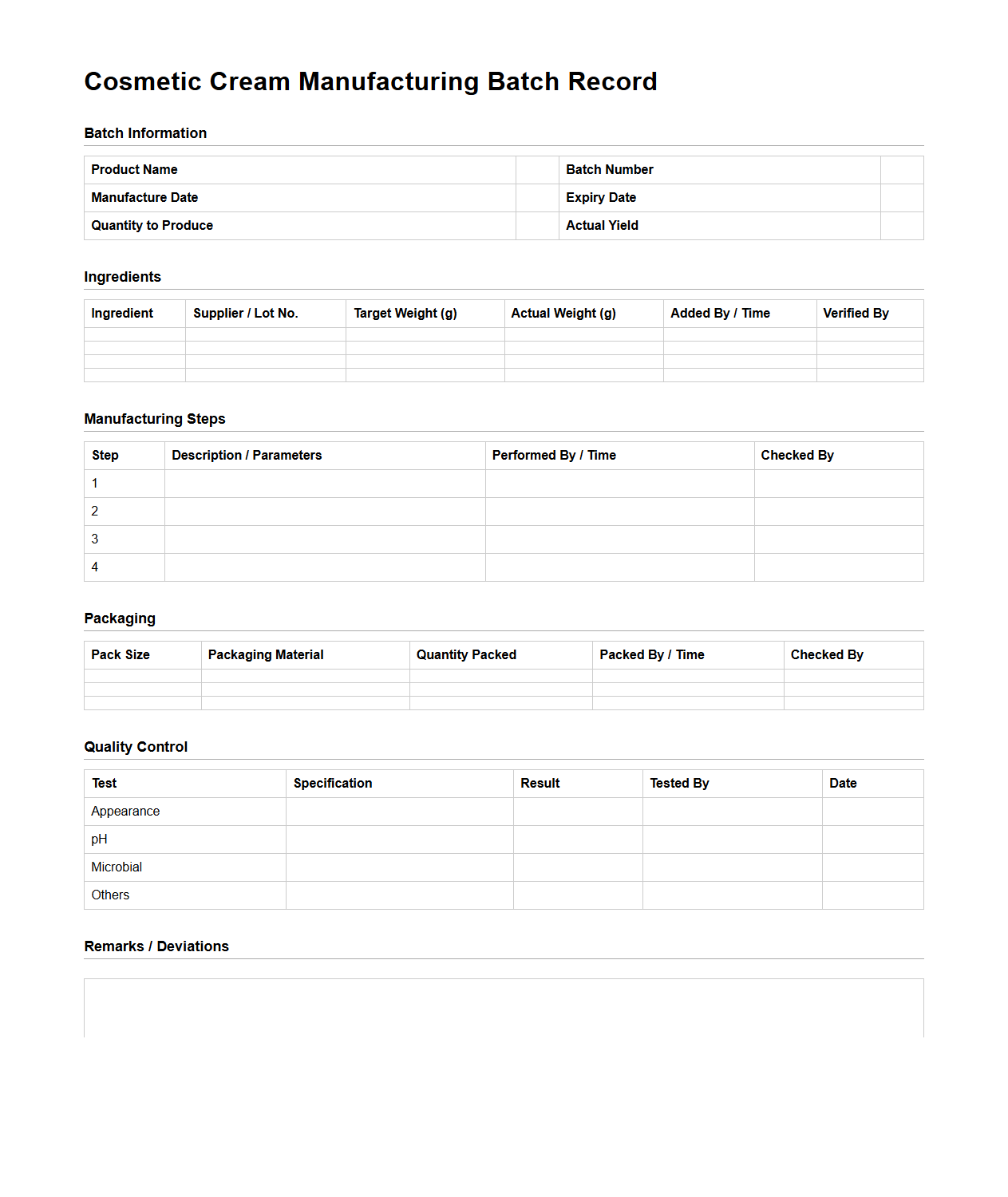
Cosmetic Cream Manufacturing Batch Record Sample
A
Cosmetic Cream Manufacturing Batch Record Sample document is a detailed template used to record all critical production data for a specific batch of cosmetic cream. It includes information such as raw material quantities, processing parameters, quality control results, and personnel involved, ensuring traceability and compliance with Good Manufacturing Practices (GMP). This document serves as a vital tool for maintaining product consistency, safety, and regulatory adherence throughout the manufacturing process.
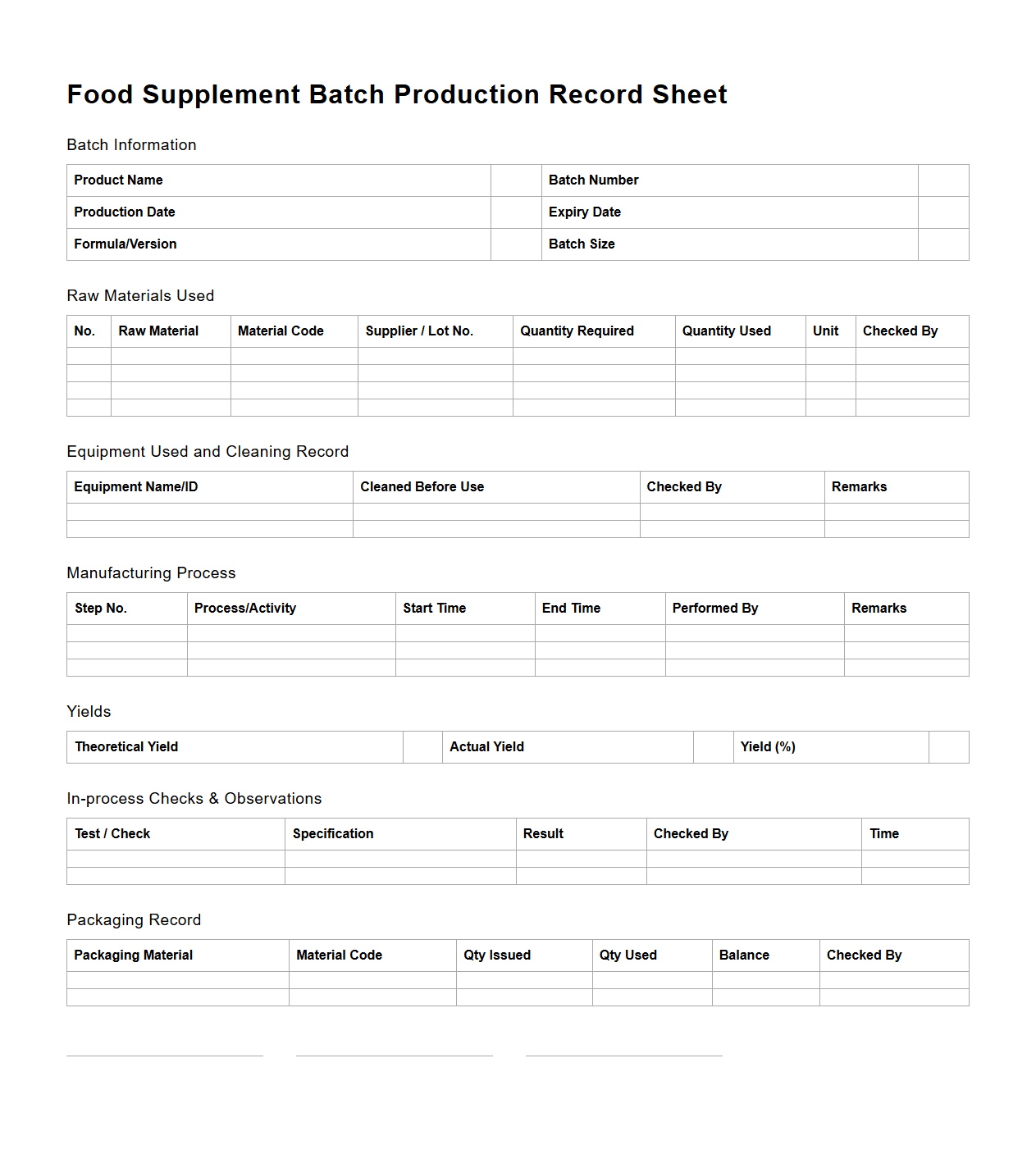
Food Supplement Batch Production Record Sheet
A
Food Supplement Batch Production Record Sheet is a critical document used in the manufacturing process to ensure traceability and quality control of each batch produced. It records detailed information such as raw material quantities, production parameters, processing times, and personnel involved, helping manufacturers maintain compliance with regulatory standards. This document serves as an essential tool for auditing, troubleshooting, and verifying the consistency and safety of the food supplement products.
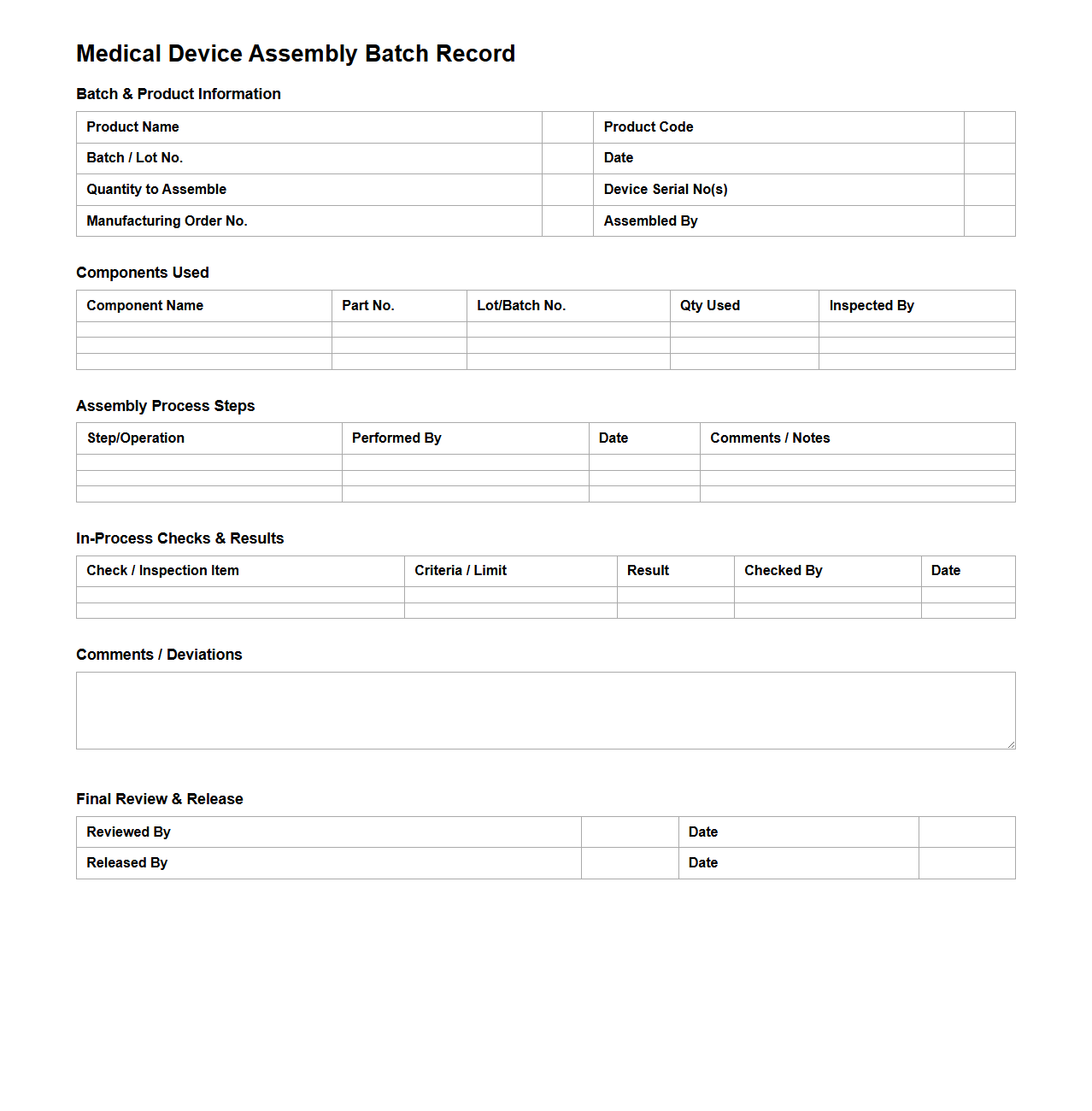
Medical Device Assembly Batch Record Format
A
Medical Device Assembly Batch Record Format document is a structured template used to systematically record the manufacturing process of medical device batches, ensuring traceability and regulatory compliance. It includes detailed information such as component lot numbers, assembly procedures, quality checks, and personnel involved in production. This document is essential for maintaining product consistency, meeting FDA and ISO standards, and facilitating audits and quality control processes.
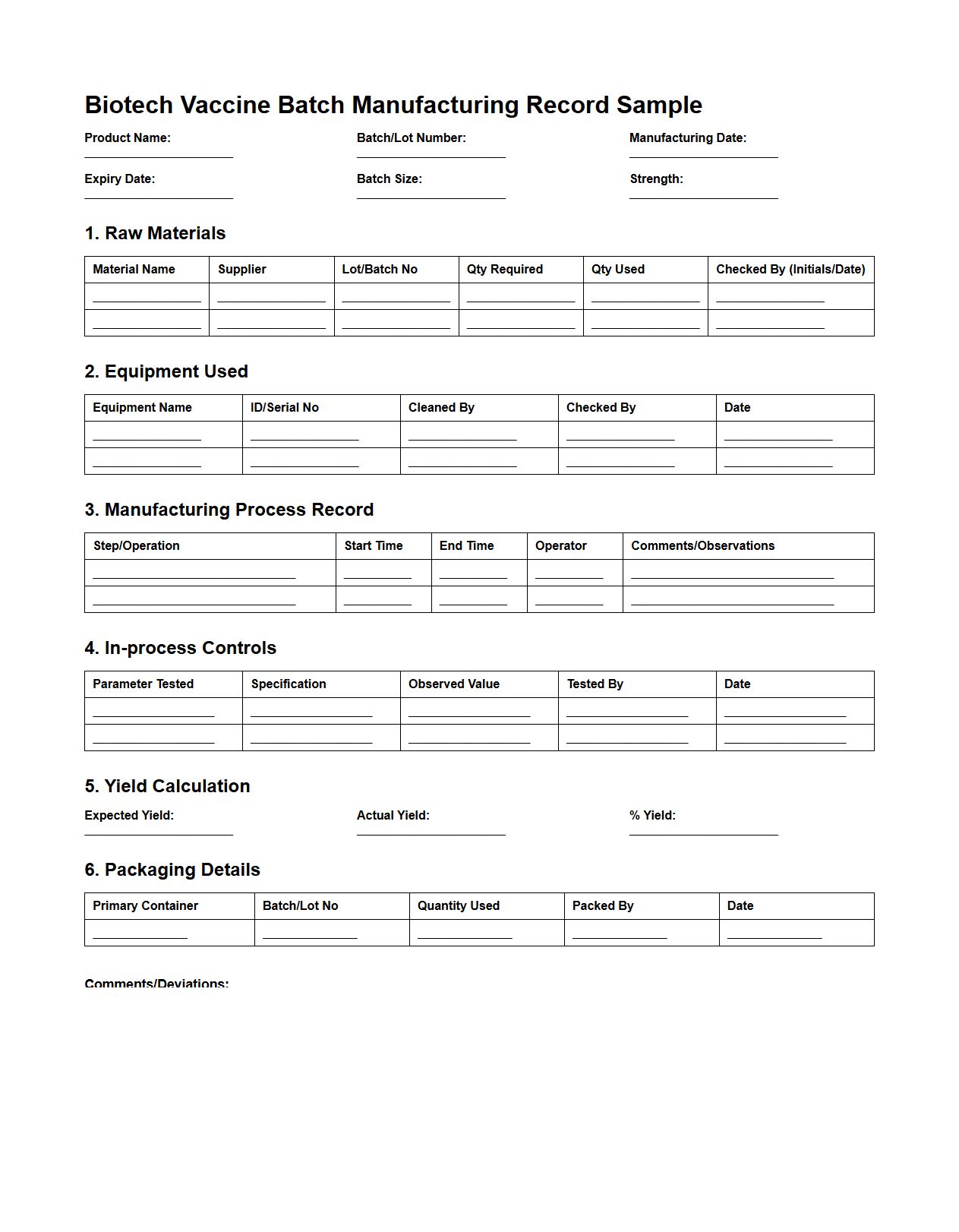
Biotech Vaccine Batch Manufacturing Record Sample
The
Biotech Vaccine Batch Manufacturing Record (BMR) Sample document is a critical record outlining detailed information about the production process of a specific vaccine batch. It includes data such as raw materials, equipment used, process parameters, quality control results, and deviation reports to ensure traceability and compliance with regulatory standards. This document serves as an essential reference for verifying consistency, safety, and efficacy in vaccine manufacturing.
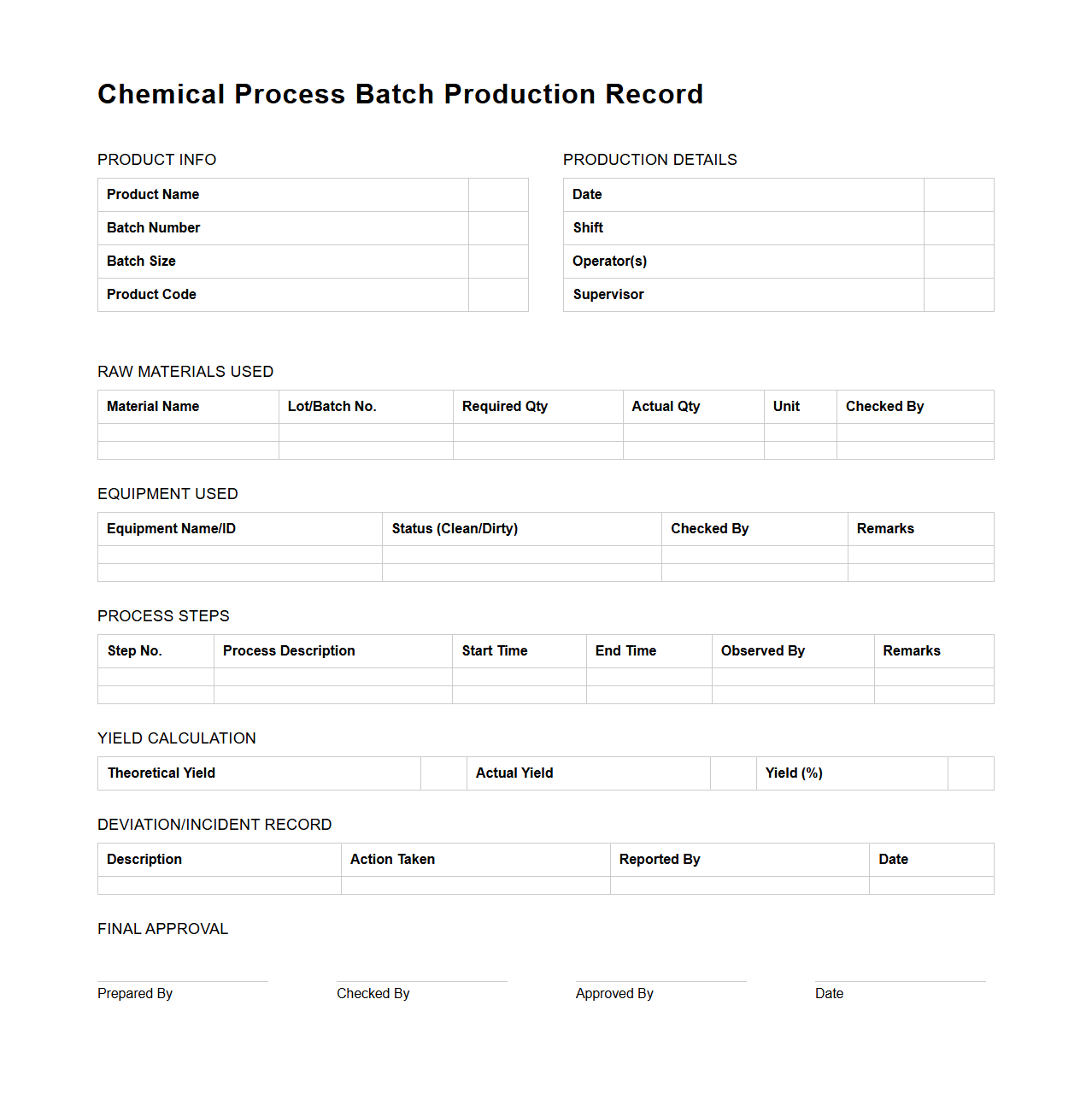
Chemical Process Batch Production Record Template
The
Chemical Process Batch Production Record Template document serves as a detailed log that captures every step, ingredient, and condition involved in producing a chemical batch. It ensures traceability, quality control, and compliance with regulatory standards by meticulously recording process parameters, raw material lot numbers, and equipment used. This template is essential for maintaining consistency and facilitating audits in chemical manufacturing operations.
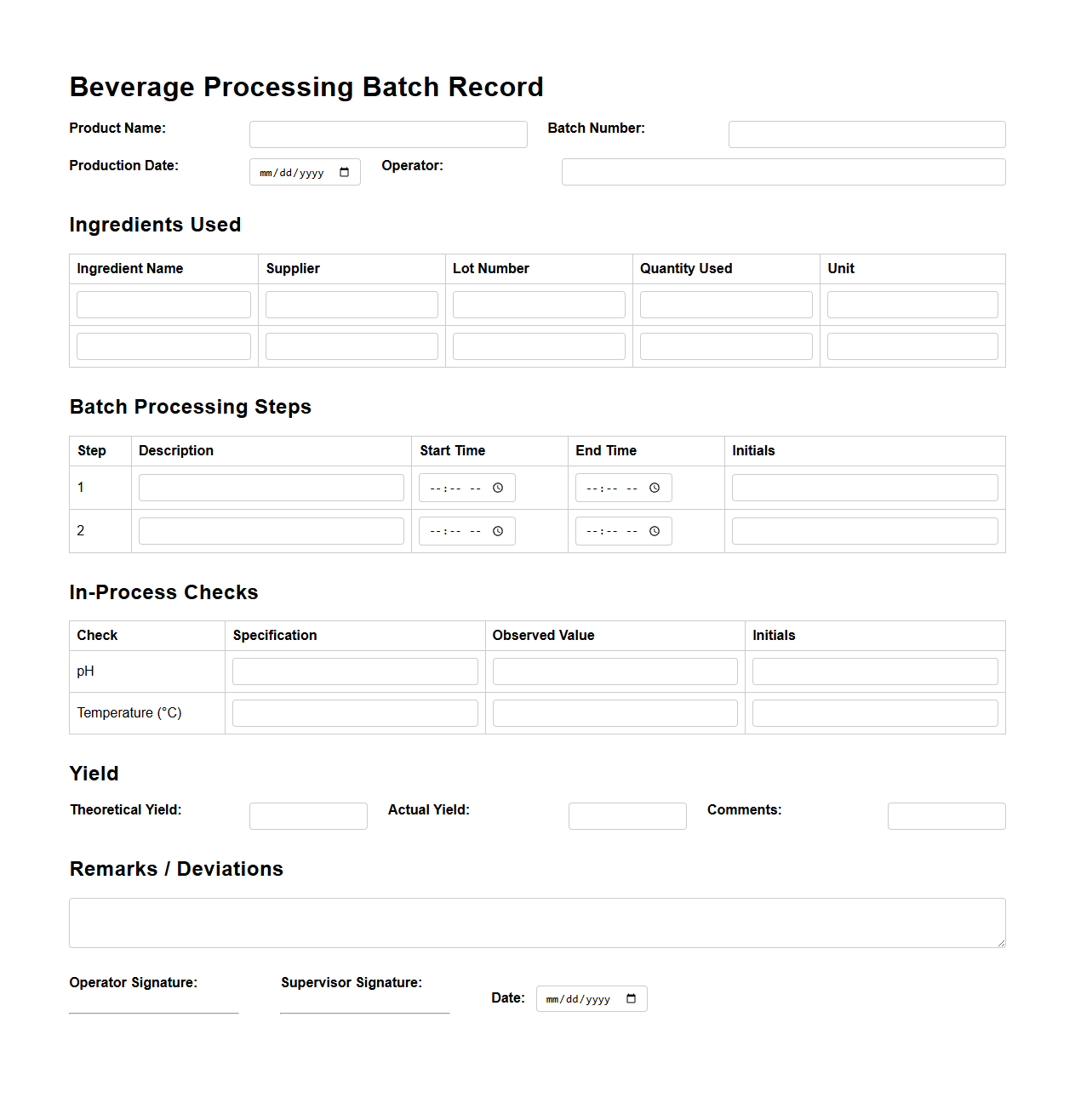
Beverage Processing Batch Record Documentation
Beverage Processing Batch Record Documentation is a critical
quality control tool that records every step of the production process, ensuring traceability and compliance with industry standards. It includes detailed information such as ingredient quantities, processing conditions, equipment used, and personnel involved. Accurate batch records help identify potential issues, maintain product consistency, and support regulatory audits in the beverage manufacturing industry.
Personal Care Product Batch Record Sample
A
Personal Care Product Batch Record Sample document is a detailed log used in the manufacturing process to track and verify the production of personal care products, ensuring consistency, quality, and regulatory compliance. It includes critical information such as raw material quantities, processing steps, equipment used, and quality control test results. This document serves as a reference for auditing, troubleshooting, and maintaining traceability throughout the production cycle.
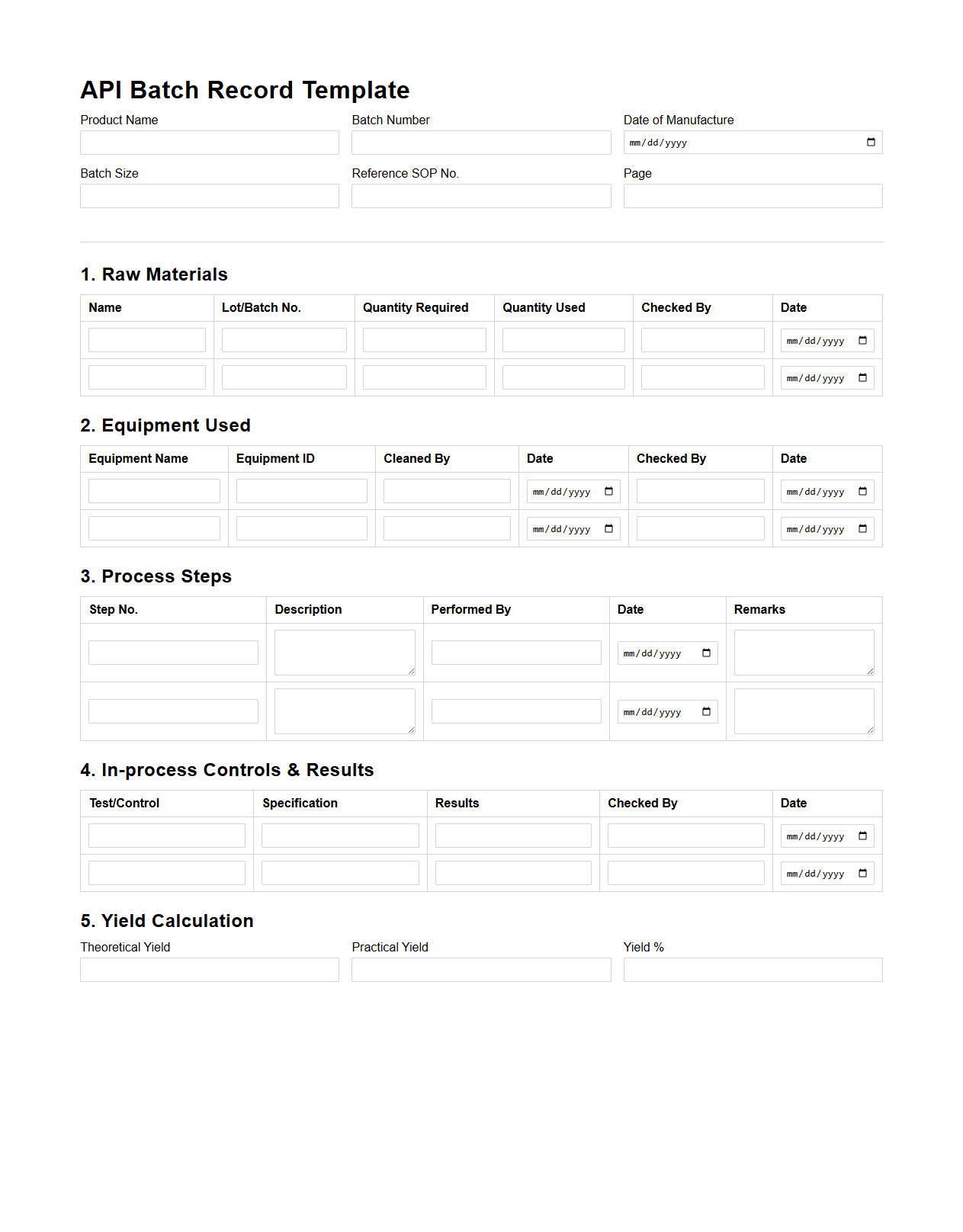
Active Pharmaceutical Ingredient Batch Record Template
The
Active Pharmaceutical Ingredient (API) Batch Record Template document is a standardized form used to meticulously record the manufacturing process and quality control data for each batch of pharmaceutical ingredient produced. This template ensures compliance with regulatory requirements by capturing critical information such as raw material specifications, equipment used, process parameters, and analytical test results. Maintaining accurate API batch records is essential for traceability, quality assurance, and facilitating audits in pharmaceutical production.
What critical information must be included in a Production Batch Record Document to ensure traceability and compliance?
A Production Batch Record Document must include detailed information such as batch numbers, manufacturing dates, and equipment identifiers. This ensures complete traceability throughout the production process and adherence to regulatory compliance. Additionally, operator signatures and process parameters are critical elements for validation and accountability.
How are deviations from the standard manufacturing process documented within the batch record?
Deviations from the standard manufacturing process are recorded in a dedicated deviation log section within the batch record. Each deviation must include the nature of the discrepancy, investigation findings, and corrective actions taken. This structured documentation supports quality assurance and regulatory audit requirements.
What controls are in place for batch record review and approval before and after production?
Batch records undergo a thorough review and approval by designated quality assurance personnel both prior to and following production. This process includes verifying completeness, accuracy, and compliance with standard operating procedures. Electronic or manual signatures are typically required to validate final batch acceptance.
Which sections of the batch record capture in-process testing and quality control results?
The in-process testing and quality control results are documented in specific testing sections of the batch record. These sections record data such as sampling times, test methods, and outcomes against predefined acceptance criteria. Accurate capture of this information ensures immediate detection of non-conformities during production.
How are raw materials and components accounted for in the Production Batch Record Document?
Raw materials and components are meticulously tracked through lot numbers, quantities used, and supplier details in the batch record. This accounting includes verification steps ensuring the correct materials are utilized per recipe specifications. Proper documentation supports inventory control and traceability for quality assurance purposes.