A Standard Operating Procedure Document Sample for Manufacturing provides a clear and detailed guideline to ensure consistent production processes and maintain quality control. It outlines step-by-step instructions for employees to follow, minimizing errors and improving efficiency. This document is essential for training new staff and meeting industry compliance standards.
Equipment Maintenance Standard Operating Procedure Template
The
Equipment Maintenance Standard Operating Procedure (SOP) Template document provides a structured framework for consistently performing maintenance tasks on machinery and equipment. It outlines specific steps, safety protocols, and quality checks to ensure optimal equipment performance and minimize downtime. This template enhances operational efficiency by standardizing procedures across maintenance teams.
Raw Material Inspection SOP Example
A
Raw Material Inspection SOP Example document outlines standardized procedures for verifying the quality and compliance of incoming raw materials in manufacturing or production processes. It ensures that materials meet specified standards to prevent defects and maintain product integrity. This document serves as a critical reference for quality control teams to consistently execute inspections and record results accurately.
Production Line Changeover SOP Sample
A
Production Line Changeover SOP Sample document outlines standardized procedures for switching manufacturing equipment from producing one product to another, ensuring minimal downtime and consistent quality. It includes detailed steps for cleaning, equipment adjustment, verification, and safety checks to streamline the transition process. This document helps manufacturers improve efficiency, reduce errors, and maintain compliance with industry standards.
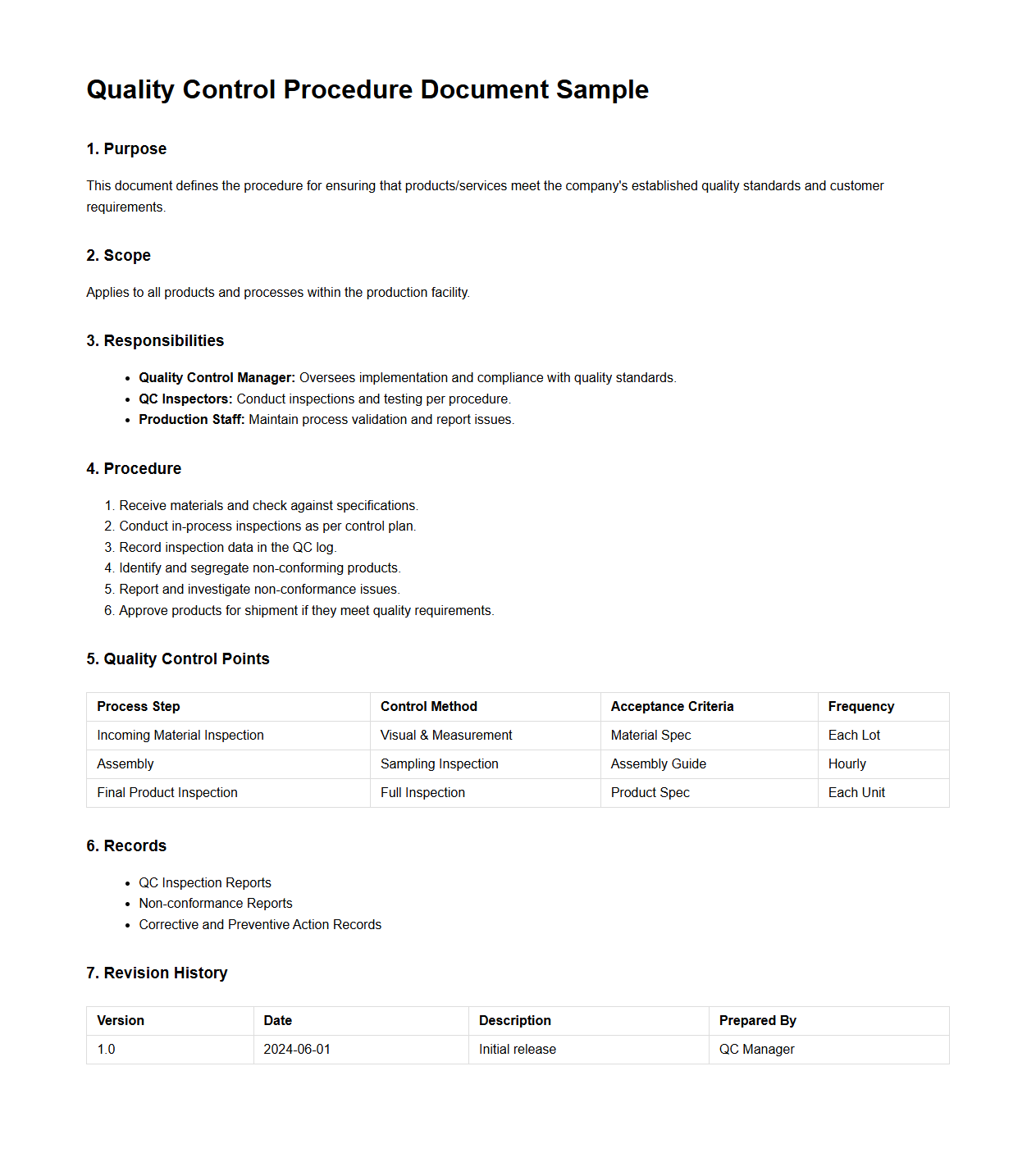
Quality Control Procedure Document Sample
A
Quality Control Procedure Document Sample outlines standardized methods and criteria used to ensure products or services meet specified quality standards. It includes step-by-step instructions, inspection checkpoints, and corrective action guidelines to maintain consistency and compliance with industry regulations. This document serves as a reference for training, audits, and continuous improvement in quality management systems.
Finished Goods Packaging SOP Template
The
Finished Goods Packaging SOP Template document serves as a standardized guideline outlining the procedures and quality standards for packaging final products. It ensures consistency in packaging processes, compliance with regulatory requirements, and protection of goods during storage and transportation. This template helps streamline operations, reduce errors, and maintain product integrity from production to delivery.
Cleaning and Sanitation SOP Example
A
Cleaning and Sanitation SOP Example document outlines standardized procedures to ensure thorough and consistent cleaning practices in various environments, such as food processing facilities or healthcare settings. It details specific steps, materials, and safety measures required to maintain hygiene standards and prevent contamination. This document serves as a critical reference for training staff and verifying compliance with regulatory requirements.
Inventory Management Procedure Sample
An
Inventory Management Procedure Sample document outlines standardized steps for tracking, controlling, and organizing stock levels within a business. It serves as a guide to ensure accurate recording, minimize discrepancies, and optimize inventory turnover rates. This document typically includes methods for receiving, storing, and issuing inventory to maintain efficient supply chain operations.
Non-Conformance Management SOP Example
The
Non-Conformance Management SOP Example document outlines standardized procedures to identify, document, and address deviations from established quality standards in processes or products. It provides clear guidelines for reporting non-conformances, conducting root cause analysis, implementing corrective actions, and tracking resolution status. This ensures consistent handling of non-conformities, minimizing impact on operations and maintaining compliance with regulatory requirements.
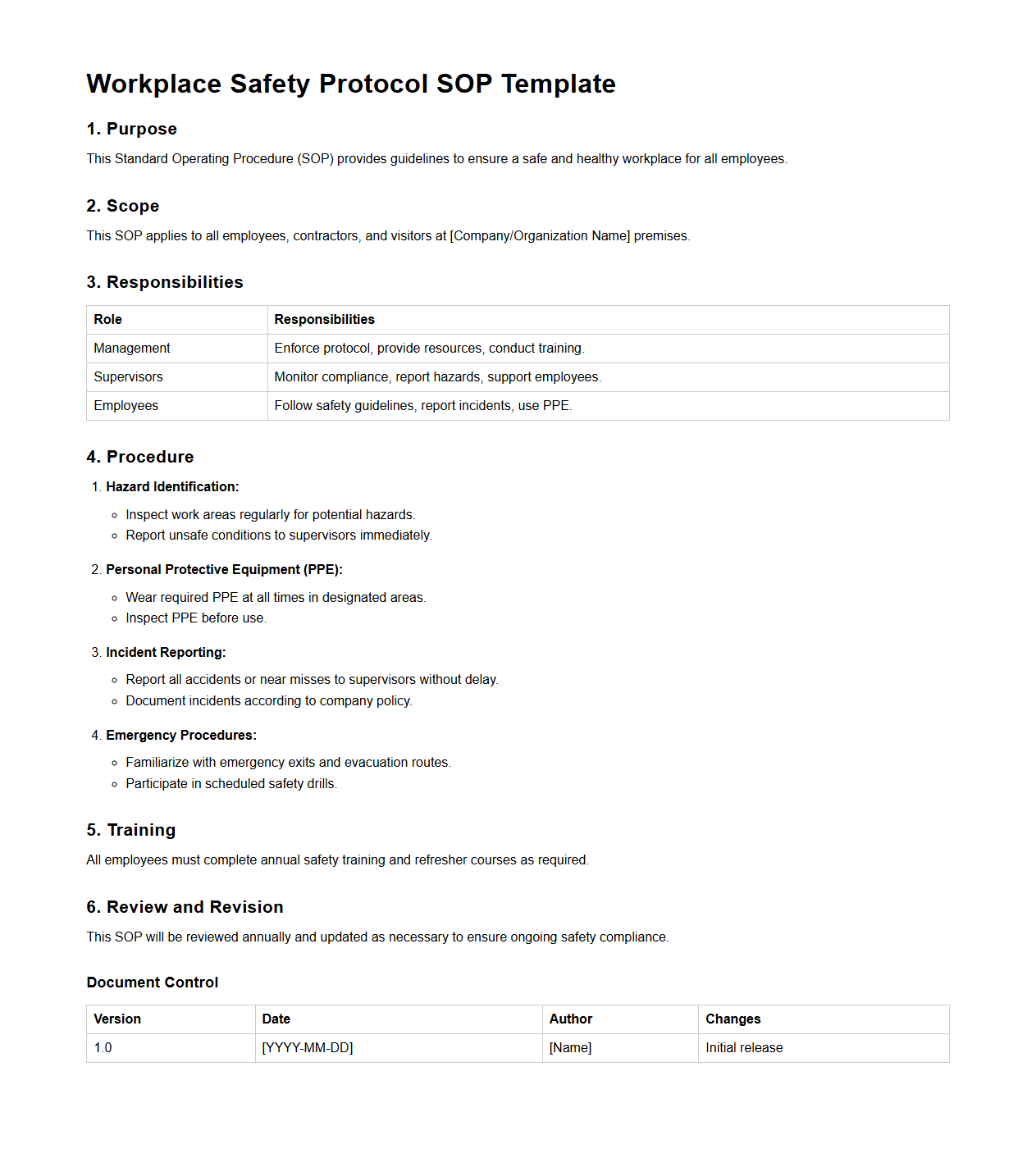
Workplace Safety Protocol SOP Template
A
Workplace Safety Protocol SOP Template document serves as a standardized framework outlining essential procedures and best practices to maintain a safe work environment. It provides clear instructions for hazard identification, risk mitigation, emergency response, and employee responsibilities, ensuring compliance with regulatory standards. This template facilitates consistent implementation of safety measures across various departments, reducing accidents and promoting organizational health.
Machine Calibration Procedure Document Sample
A
Machine Calibration Procedure Document Sample provides a standardized template outlining the step-by-step process to accurately calibrate machinery, ensuring optimal performance and compliance with industry standards. It includes detailed instructions on the calibration intervals, tools required, measurement methods, and acceptance criteria to maintain consistency and precision. This document serves as a critical reference to minimize equipment errors and uphold quality control in manufacturing or laboratory environments.
What are the primary objectives outlined in this Standard Operating Procedure (SOP) document for manufacturing processes?
The primary objectives of the SOP are to ensure consistent, reliable, and efficient manufacturing operations. It aims to standardize processes to minimize errors and enhance product quality. Additionally, the SOP focuses on compliance with regulatory requirements and safety standards to protect both staff and product integrity.
Which critical steps must operators follow according to the SOP before initiating production activities?
Operators must perform a thorough equipment inspection and ensure all machinery is calibrated correctly before starting production. Verifying raw materials for quality and proper labeling is essential to maintain process integrity. Finally, operators must review and understand the batch production record to confirm all preparation steps are completed accurately.
What specific quality control measures are described in the SOP to ensure product consistency?
The SOP mandates regular in-process quality checks at critical stages to detect any deviations early. It specifies using validated testing methods for raw materials, intermediates, and final products to maintain quality standards. Documentation of all quality control activities is required to provide traceability and support continuous improvement.
How does the SOP document define roles and responsibilities for staff involved in the manufacturing process?
The SOP clearly assigns specific roles and responsibilities to ensure accountability at each step of the manufacturing process. It delineates tasks for operators, supervisors, and quality control personnel to prevent overlap and gaps. Moreover, it requires training and competency verification for all staff before they can perform their duties.
What procedures are detailed in the SOP for reporting and managing deviations or incidents during production?
The SOP outlines a structured deviation reporting process requiring immediate notification to supervisors and documentation of the event. It includes guidelines for root cause analysis and corrective actions to prevent recurrence. All incidents must be reviewed and approved by quality assurance before resuming normal production activities.
More Manufacturing Templates