A Quality Control Inspection Document Sample for Manufacturing outlines the essential criteria and procedures to ensure product standards are consistently met. It typically includes detailed checklists, measurement data, and defect tracking to facilitate thorough inspection processes. Using this document helps maintain production quality and supports compliance with industry regulations.
Incoming Raw Material Inspection Report Template
An
Incoming Raw Material Inspection Report Template document is a standardized form used by quality control teams to systematically evaluate and record the quality and compliance of raw materials upon receipt. It captures essential data such as supplier information, material specifications, batch numbers, inspection criteria, test results, and any deviations or defects identified during the inspection process. This report ensures that only materials meeting predefined quality standards proceed to production, reducing the risk of defects and maintaining overall product integrity.
In-Process Quality Inspection Checklist
An
In-Process Quality Inspection Checklist document serves as a critical tool to monitor and verify product quality during various stages of manufacturing or production. It systematically outlines specific inspection criteria, measurement standards, and acceptable tolerance levels to ensure compliance with quality requirements. By facilitating real-time defect identification and corrective actions, this checklist enhances overall process control and reduces the risk of non-conformities.
Final Product Quality Control Inspection Form
The
Final Product Quality Control Inspection Form document is used to systematically evaluate the quality and compliance of finished goods before release. It includes detailed checkpoints for product specifications, defect identification, and adherence to industry standards. This form ensures that only products meeting predetermined quality criteria reach customers, reducing returns and enhancing brand reputation.
Production Line Quality Audit Report Sample
A
Production Line Quality Audit Report Sample document serves as a detailed record that evaluates the efficiency, compliance, and overall quality standards of a manufacturing production line. It typically includes key performance metrics, non-conformance issues, corrective actions, and recommendations to ensure adherence to industry regulations and internal quality benchmarks. This report aids organizations in identifying process improvements and maintaining consistent product quality throughout the production cycle.
Manufacturing Defect Tracking Log Template
A
Manufacturing Defect Tracking Log Template document is a structured tool designed to systematically record, monitor, and analyze defects identified during the production process. This template typically includes fields for defect description, occurrence date, responsible department, root cause analysis, and corrective actions taken, facilitating efficient quality control and continuous improvement. Using this log enhances traceability of issues, reduces recurrence of defects, and supports compliance with industry standards.
First Article Inspection Report Example
A
First Article Inspection Report Example document serves as a comprehensive record verifying that a manufactured product meets all specified design, material, and performance requirements during initial production. It includes detailed measurements, test results, and compliance assessments to ensure quality standards are met before full-scale manufacturing begins. This report is crucial for identifying discrepancies early, reducing defects, and maintaining consistency in mass production.
Packaging Quality Inspection Checklist
A
Packaging Quality Inspection Checklist document serves as a critical tool to ensure packaging meets required standards for protection, labeling, and compliance. It systematically evaluates factors like material integrity, sealing quality, print accuracy, and regulatory adherence to minimize defects and enhance product safety. This checklist aids quality control teams in maintaining consistent packaging performance and customer satisfaction across the supply chain.
Supplier Quality Assessment Document
A
Supplier Quality Assessment Document is a critical tool used to evaluate and verify the quality standards of suppliers before and during the procurement process. It typically contains detailed criteria such as production capabilities, compliance with industry standards, and quality control processes to ensure suppliers meet required specifications. This document helps organizations minimize risks, improve supplier reliability, and maintain consistent product quality throughout the supply chain.
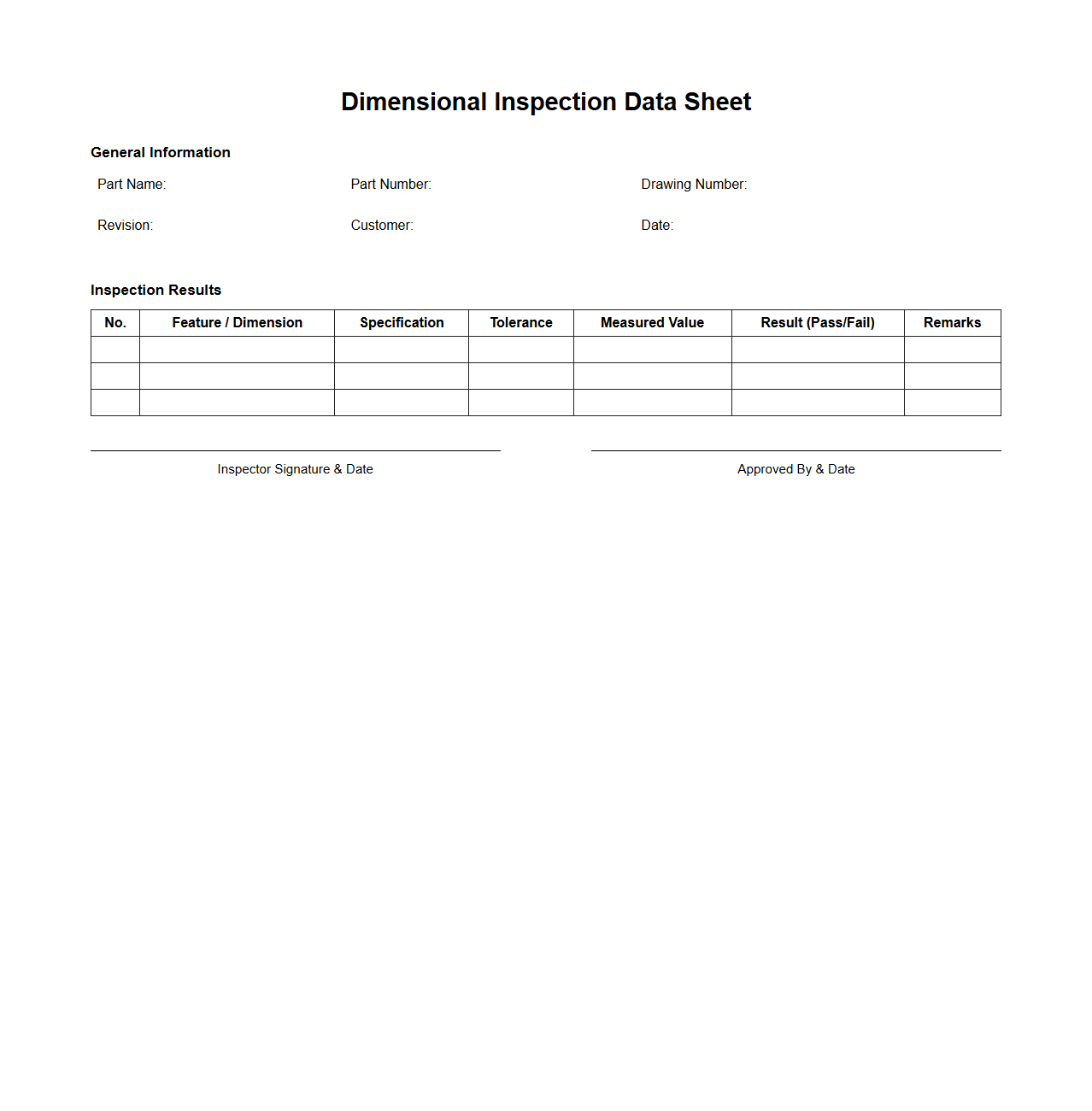
Dimensional Inspection Data Sheet
The
Dimensional Inspection Data Sheet is a critical quality control document used to record precise measurements of manufactured parts or components against specified engineering tolerances. This sheet captures detailed data such as length, width, height, diameter, and other geometric dimensions to ensure compliance with design specifications. Accurate dimensional inspection data aids in identifying deviations, maintaining product consistency, and supporting process improvements in manufacturing.
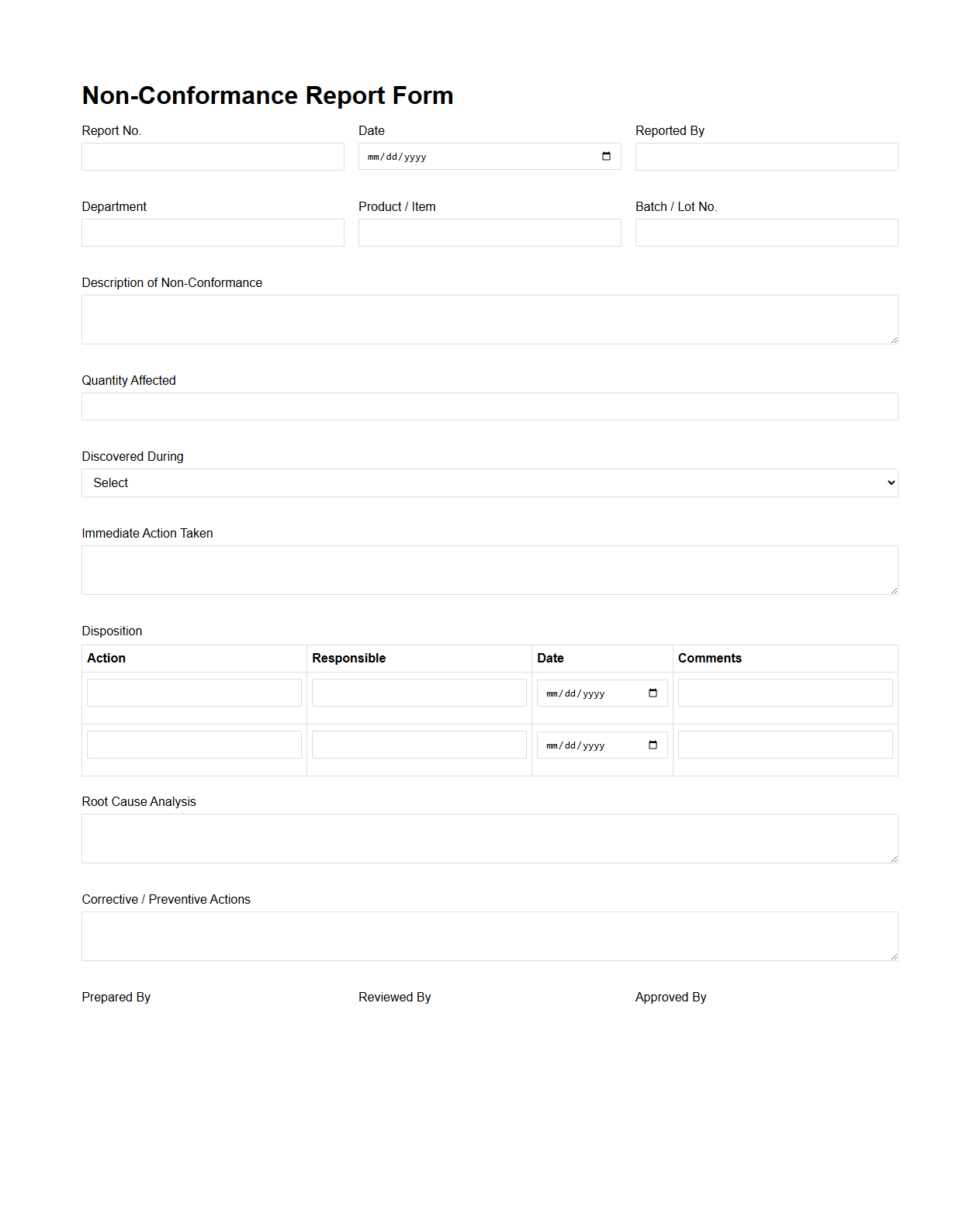
Non-Conformance Report Form for Manufacturing
A
Non-Conformance Report (NCR) form in manufacturing is a critical document used to identify, record, and address deviations from specified standards or requirements in production processes. It captures detailed information about defects, non-compliant materials, or procedural errors to facilitate root cause analysis and corrective actions. This form ensures accountability, continuous improvement, and adherence to quality management systems such as ISO 9001.
Key Components of a Quality Control Inspection Document
A Quality Control Inspection Document must include product specifications, inspection criteria, and detailed records of measurements. It should also list the inspection methods and tools used to verify compliance with standards. Additionally, operator details and date/time stamps are essential for accountability and process validation.
Ensuring Traceability of Inspected Products and Batches
The document helps ensure traceability by recording unique batch numbers, serial numbers, and production dates for each product. Barcodes or QR codes are often incorporated for efficient tracking through the supply chain. This detailed recording facilitates quick retrieval and root cause analysis if defects arise.
Criteria for Acceptance or Rejection of Items
The inspection document outlines acceptance criteria based on tolerance limits derived from product standards or customer requirements. Measurements outside these limits trigger rejection or rework protocols. Visual inspections, dimensional checks, and functional tests are commonly used to evaluate product conformity.
Documentation and Handling of Nonconforming Products
Nonconforming products are documented with detailed descriptions of the defect and associated batch information. The inspection report includes disposition instructions, such as rework, scrap, or return to the supplier. This systematic approach ensures appropriate handling and prevents defective items from reaching customers.
Referenced Quality Standards and Regulatory Compliance
This document sample aligns with prominent quality standards like ISO 9001 and industry-specific regulations such as FDA or CE requirements. Compliance ensures that the inspection process meets international quality benchmarks and legal obligations. Regular updates are made to incorporate changes in regulatory frameworks.
More Manufacturing Templates