A Batch Production Record Document Sample for Pharmaceutical Manufacturing is a critical template that details the step-by-step manufacturing process to ensure consistency and compliance with regulatory standards. It includes information on raw materials, equipment settings, personnel involved, and quality control tests to maintain product integrity. This document serves as a vital reference for traceability and validation during pharmaceutical production audits.
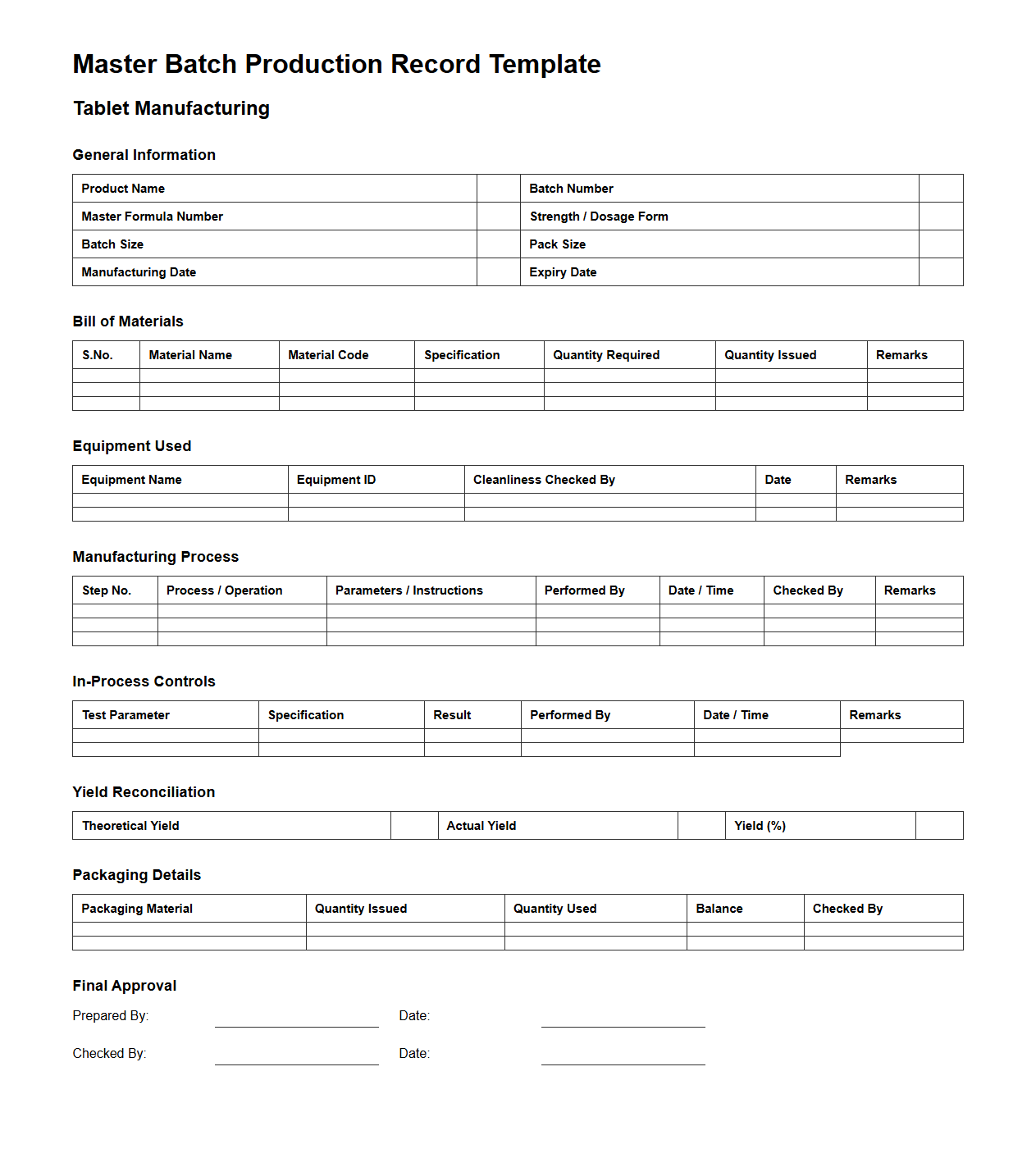
Master Batch Production Record Template for Tablet Manufacturing
The
Master Batch Production Record Template for tablet manufacturing is a detailed document that outlines the standardized procedures, ingredients, equipment, and specifications required for producing a specific batch of tablets. It ensures consistency, quality control, and regulatory compliance during the manufacturing process by providing step-by-step instructions and critical parameters. This template serves as a crucial reference for production teams and auditors to maintain traceability and validate adherence to Good Manufacturing Practices (GMP).
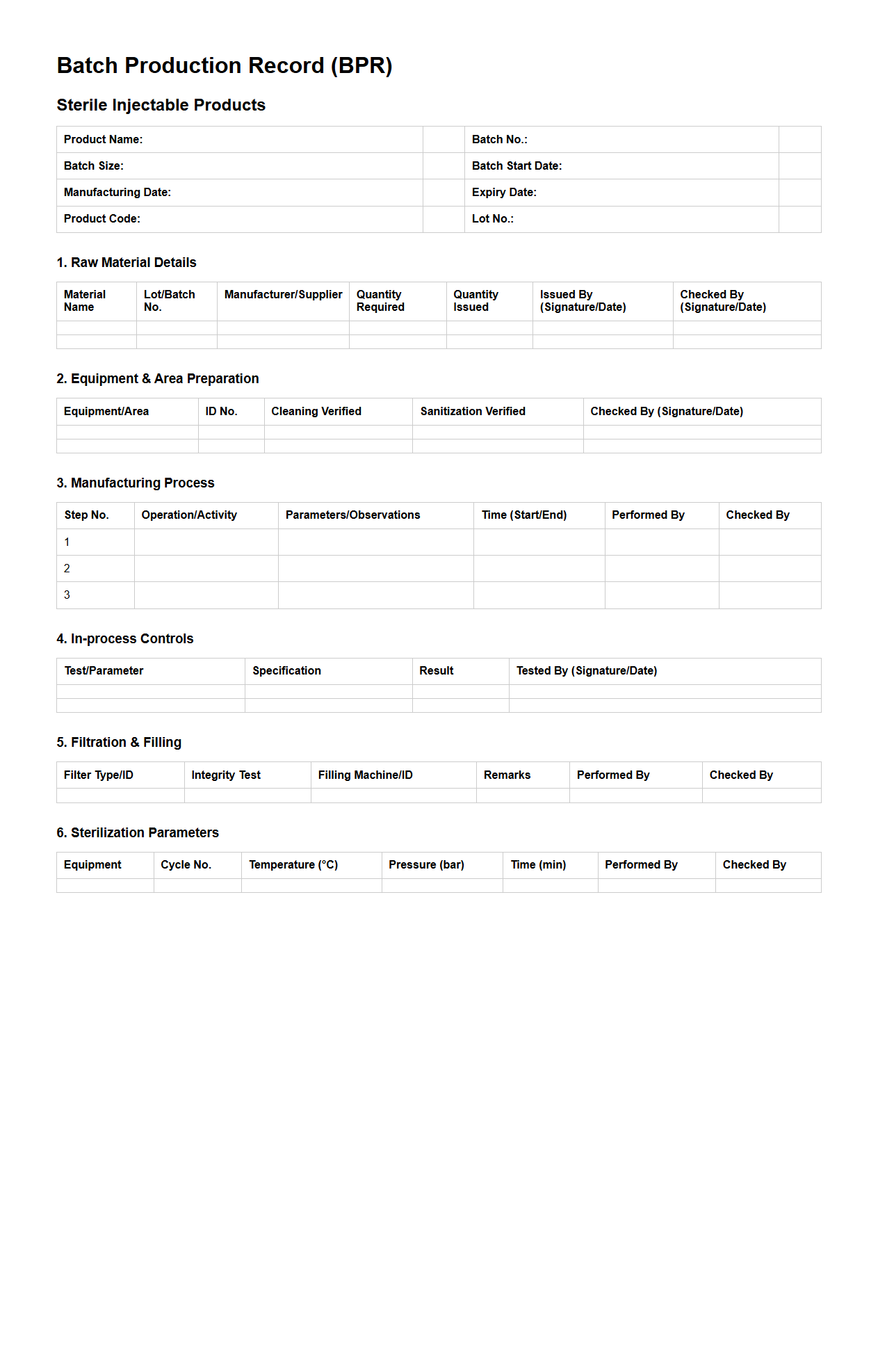
Batch Production Record Example for Sterile Injectable Products
A
Batch Production Record Example for Sterile Injectable Products document serves as a comprehensive template detailing the step-by-step manufacturing process, quality control measures, and equipment used in producing sterile injectables. It ensures compliance with regulatory standards by documenting critical parameters such as sterilization methods, formulation details, and environmental monitoring results. This record facilitates traceability, consistency, and accountability in pharmaceutical production to maintain product safety and efficacy.
BPR Document Sample for Oral Solid Dosage Forms
A
BPR Document Sample for Oral Solid Dosage Forms provides a standardized template outlining the necessary procedural and compliance requirements for manufacturing tablets, capsules, and powders. It includes detailed process steps, quality control measures, and regulatory guidelines to ensure consistent product quality and safety. This document serves as a critical reference for pharmaceutical companies aiming to meet Good Manufacturing Practices (GMP) standards.
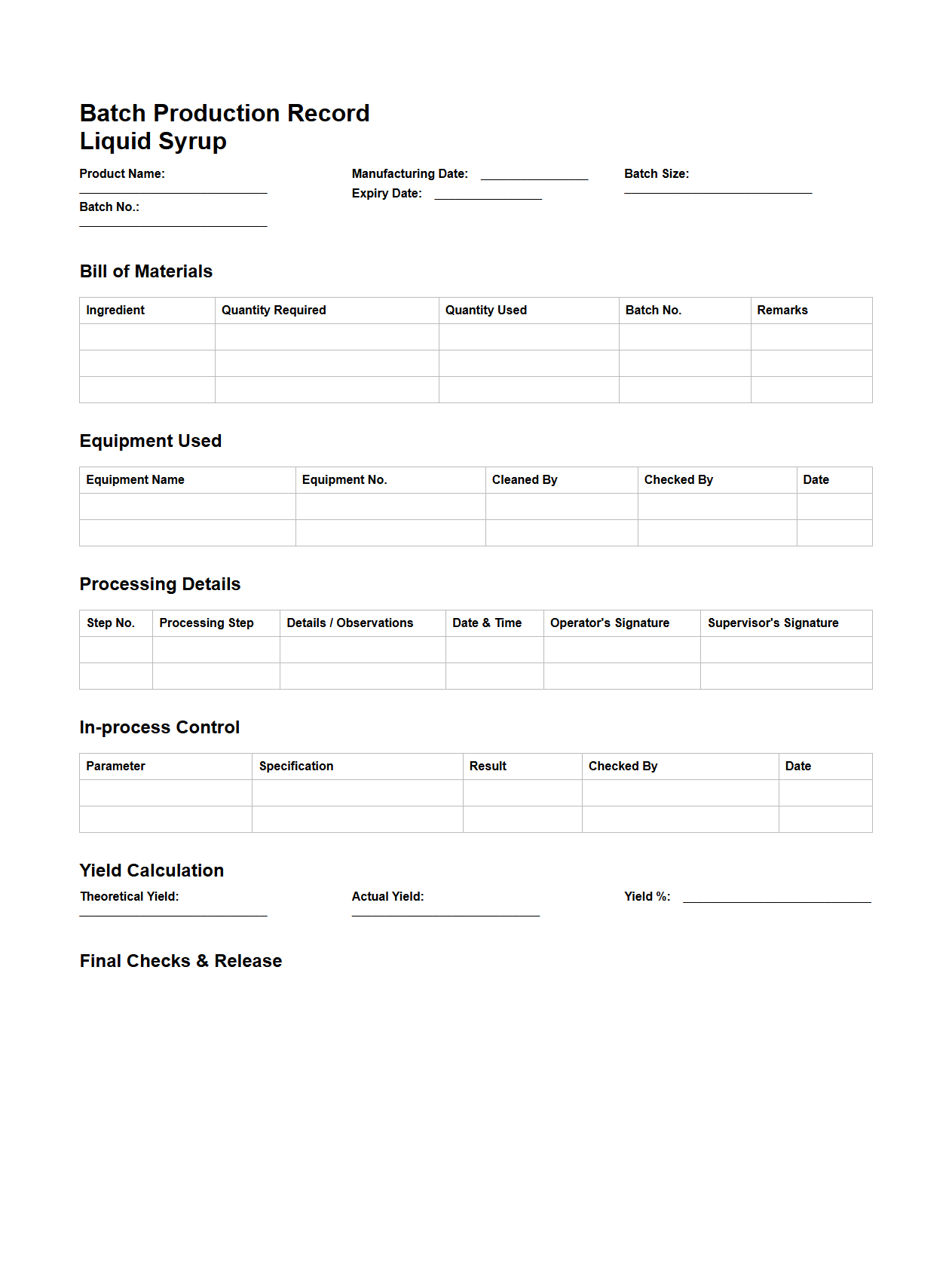
Batch Production Record Format for Liquid Syrup Production
The
Batch Production Record Format for Liquid Syrup Production is a detailed document template used to systematically record every step of the manufacturing process for liquid syrups. It includes critical data such as raw material specifications, quantity used, processing parameters, equipment employed, production dates, and quality control checks to ensure consistency and compliance with regulatory standards. This format serves as a vital tool for traceability, quality assurance, and regulatory audits in pharmaceutical or food manufacturing environments.
Sample Batch Manufacturing Record for Topical Creams
A
Sample Batch Manufacturing Record for Topical Creams document provides a detailed blueprint for the production process of a specific batch, ensuring consistency and quality control. It includes critical information such as ingredient quantities, mixing instructions, equipment used, and batch identification details. This record is essential for traceability, regulatory compliance, and maintaining standard operating procedures in pharmaceutical manufacturing.
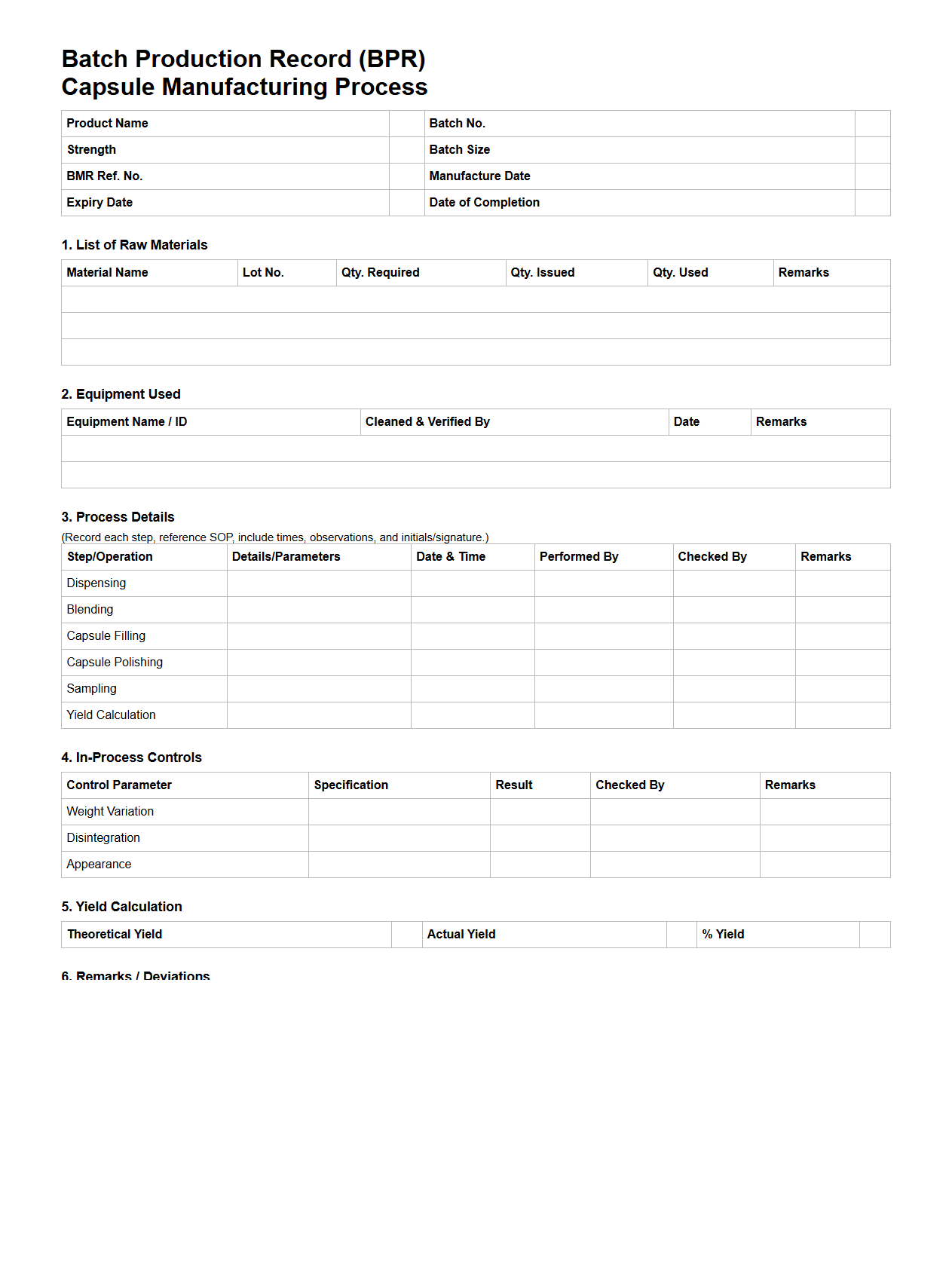
BPR Template for Capsule Manufacturing Process
A
BPR Template for Capsule Manufacturing Process document serves as a standardized framework to outline detailed steps, responsibilities, and quality controls essential for consistent capsule production. It ensures compliance with regulatory standards such as GMP and facilitates process optimization by documenting critical parameters like raw material specifications, machine settings, and in-process inspections. This template aids manufacturers in maintaining product quality, traceability, and operational efficiency throughout the capsule formulation to final packaging stages.
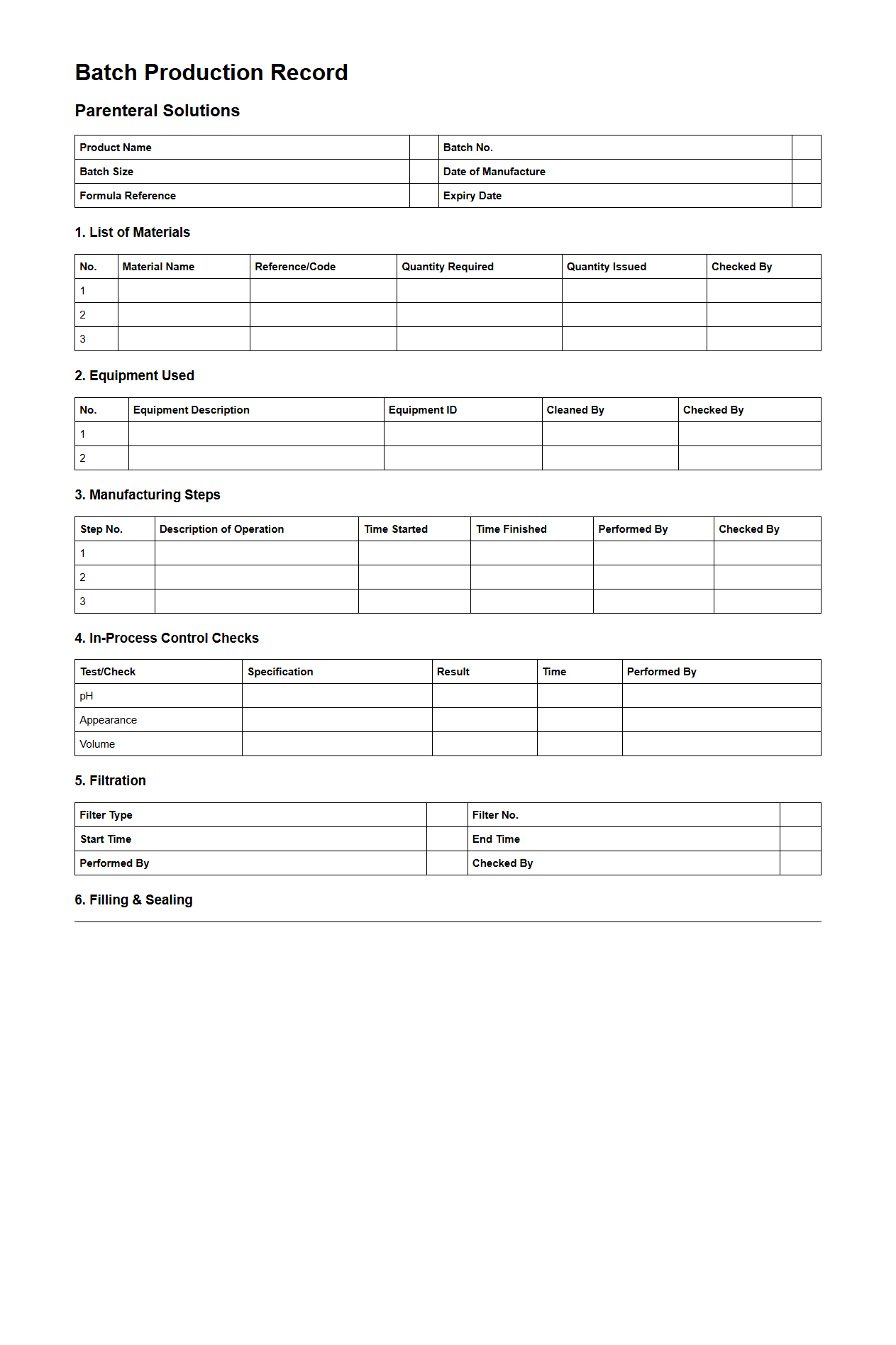
Batch Production Record Example for Parenteral Solutions
A
Batch Production Record Example for Parenteral Solutions document serves as a detailed template that outlines each step in the manufacturing process of a specific batch, ensuring compliance with regulatory standards. It includes critical data such as ingredient weights, equipment used, personnel involved, and quality control checkpoints to maintain product safety and consistency. This record is essential for traceability, process validation, and effective quality assurance in pharmaceutical production.
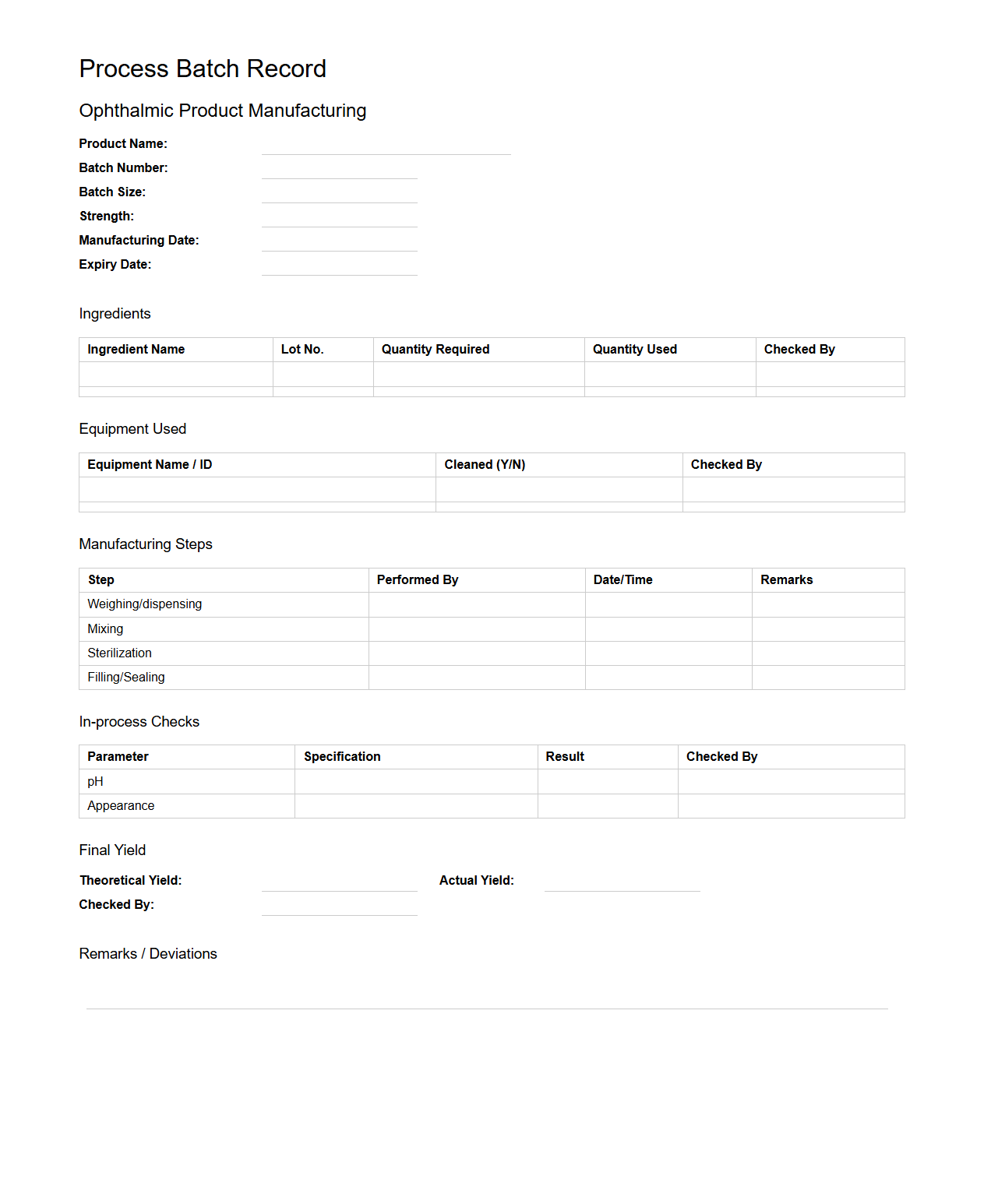
Process Batch Record for Ophthalmic Product Manufacturing
A
Process Batch Record for Ophthalmic Product Manufacturing is a detailed document that outlines each step involved in the production of eye care products, ensuring consistency and compliance with regulatory standards. It includes critical information such as ingredient quantities, equipment used, processing parameters, and quality control checkpoints to maintain product safety and efficacy. This record serves as an essential tool for traceability, batch verification, and validation in pharmaceutical manufacturing.
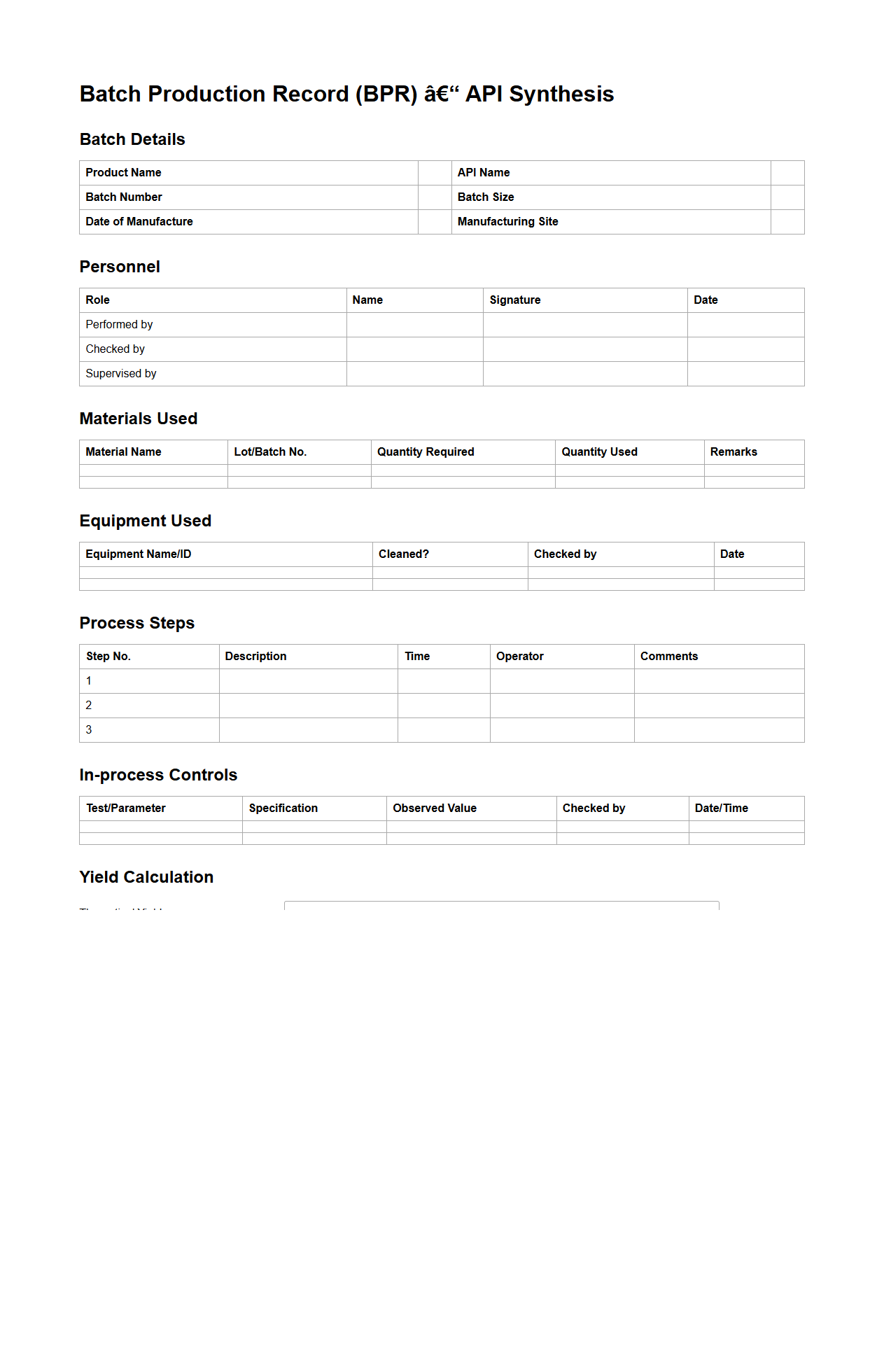
Batch Production Documentation for API Synthesis
Batch Production Documentation for API Synthesis ensures comprehensive recording of each step in the active pharmaceutical ingredient manufacturing process. This documentation includes detailed information on raw materials, equipment used, process parameters, in-process controls, and batch-specific quality data. Maintaining accurate
Batch Production Documentation is critical for regulatory compliance, traceability, and quality assurance in pharmaceutical manufacturing.
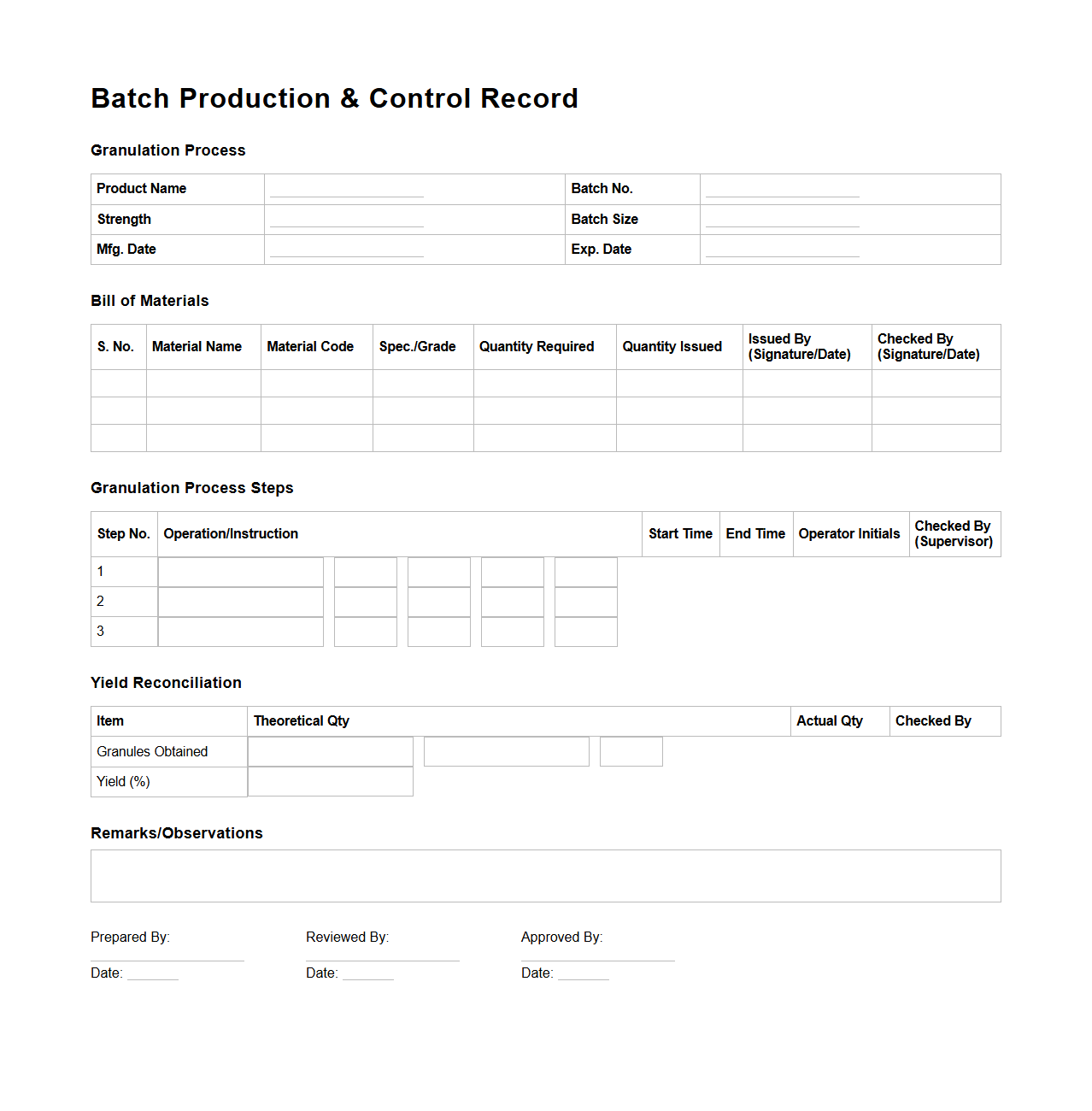
Batch Production & Control Record for Granulation Process
The
Batch Production & Control Record for the Granulation Process document serves as a detailed blueprint ensuring consistent manufacturing of granulated pharmaceutical products by documenting every step, ingredient quantity, and process parameter. It provides a traceable record to verify compliance with quality standards and facilitates troubleshooting, validation, and regulatory audits. This document is essential for maintaining process integrity and ensuring product uniformity across batches.
What critical information must be included in a Batch Production Record (BPR) for pharmaceutical manufacturing?
A Batch Production Record (BPR) must include detailed information about the raw materials, quantities, and equipment used in the manufacturing process. It should document the step-by-step manufacturing procedure, including processing times and conditions. This ensures consistency, quality control, and facilitates troubleshooting if issues arise.
How does a BPR ensure traceability and compliance in the production process?
The BPR maintains traceability by recording lot numbers, batch codes, and operator details for each stage of production. It provides an audit trail that supports compliance with regulatory standards like GMP (Good Manufacturing Practice). By capturing every relevant detail, the BPR minimizes errors and helps verify that the product meets established quality specifications.
What sections and data fields are mandatory in a standard BPR document sample?
A standard BPR must include sections such as batch identification, raw material details, equipment logs, processing parameters, and quality control results. Mandatory data fields include batch number, manufacturing date, operator signatures, and validation checklists. These elements ensure a complete and transparent record of the entire production cycle.
Which signatures and approvals are required on a completed BPR for regulatory acceptance?
A completed BPR requires signatures and approvals from the production supervisor, quality control personnel, and sometimes a quality assurance representative. These signatures confirm that the manufacturing process was performed according to protocol and all quality checks were passed. Regulatory bodies often require this chain of authorization to verify compliance and product integrity.
How does a BPR document sample support deviation and corrective action recording during batch production?
The BPR includes dedicated sections for documenting any deviations from standard procedures, detailing the nature of the issue and its impact. It also records corrective actions taken and their effectiveness to prevent recurrence. This systematic approach helps in continuous quality improvement and regulatory compliance during batch production.
More Manufacturing Templates