A Batch Record Document Sample for Pharmaceutical Manufacturing serves as a detailed template outlining each step in the drug production process. It ensures compliance with regulatory standards by documenting raw materials, equipment used, and quality control measures. This sample helps maintain consistency, traceability, and accountability throughout the manufacturing batch cycle.
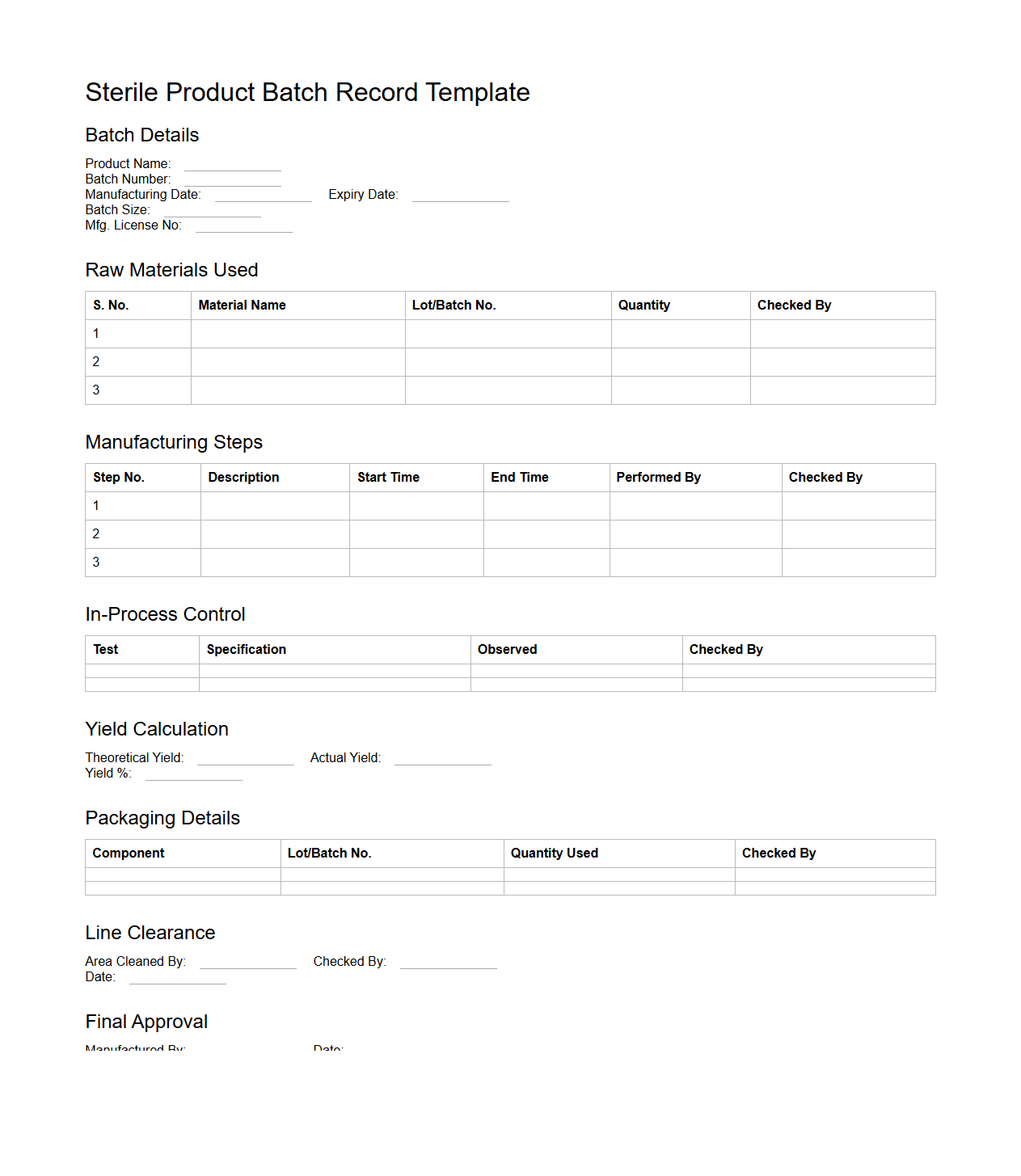
Sterile Product Batch Record Template
A
Sterile Product Batch Record Template document is a standardized form used in pharmaceutical manufacturing to ensure consistent and compliant production of sterile products. It captures detailed information on raw materials, equipment, procedures, environmental conditions, and quality control measures throughout each batch's production cycle. This template helps maintain regulatory compliance, traceability, and product safety by providing a comprehensive record of manufacturing activities.
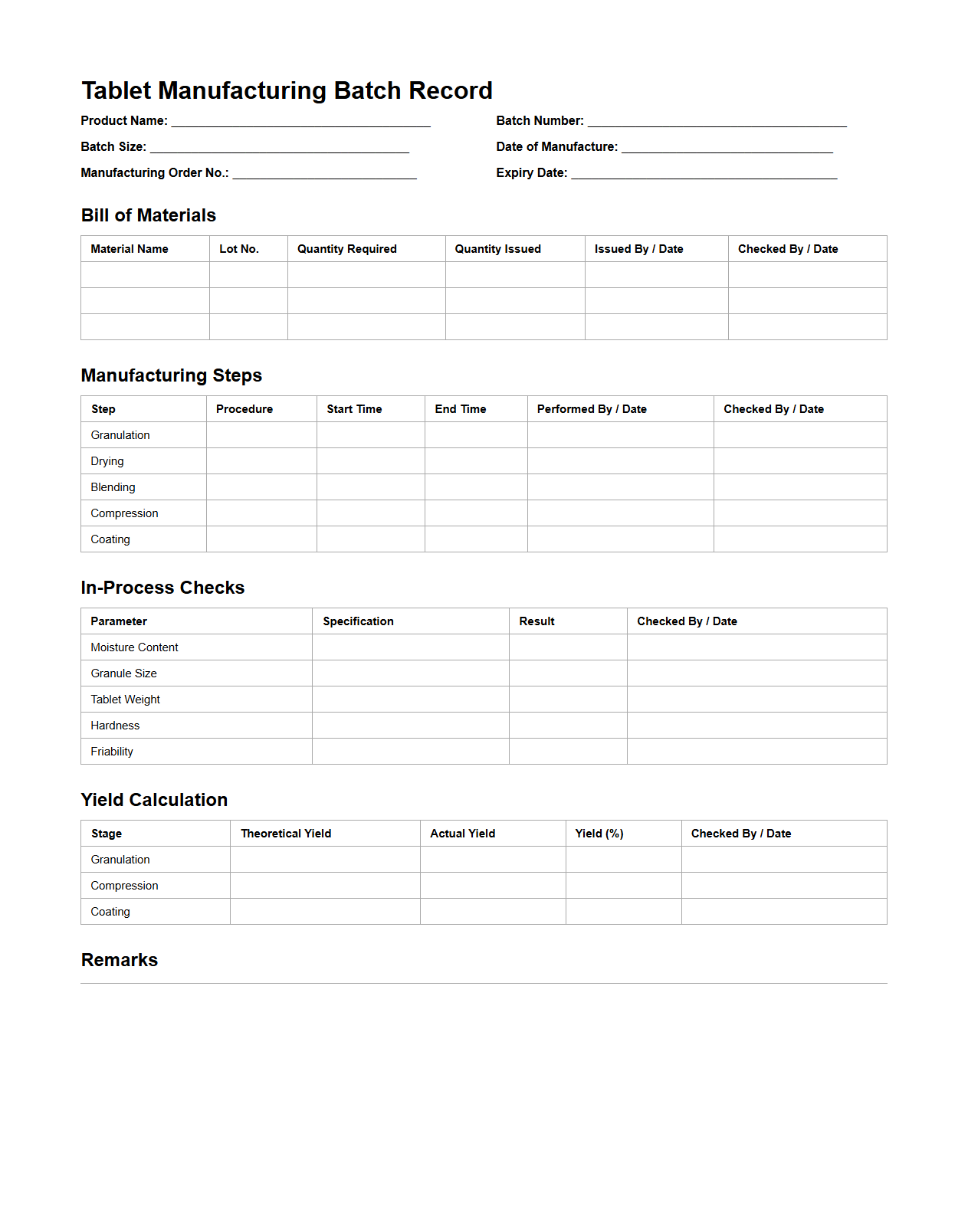
Tablet Manufacturing Batch Record Sample
A
Tablet Manufacturing Batch Record Sample document serves as a comprehensive template detailing each step in the production process of pharmaceutical tablets, ensuring consistent quality and compliance with regulatory standards. It includes critical information such as raw material specifications, equipment used, processing parameters, and quality control results to facilitate traceability and accountability. This document is essential for validating manufacturing practices and aids in troubleshooting production issues by providing a clear audit trail.
Production Batch Processing Record Form
The
Production Batch Processing Record Form is a critical document used in manufacturing to systematically capture all details of a production batch. It tracks batch-specific data such as raw materials used, process parameters, equipment settings, and quality control results, ensuring traceability and compliance with industry standards. This form plays an essential role in maintaining product consistency, facilitating audits, and supporting regulatory requirements.
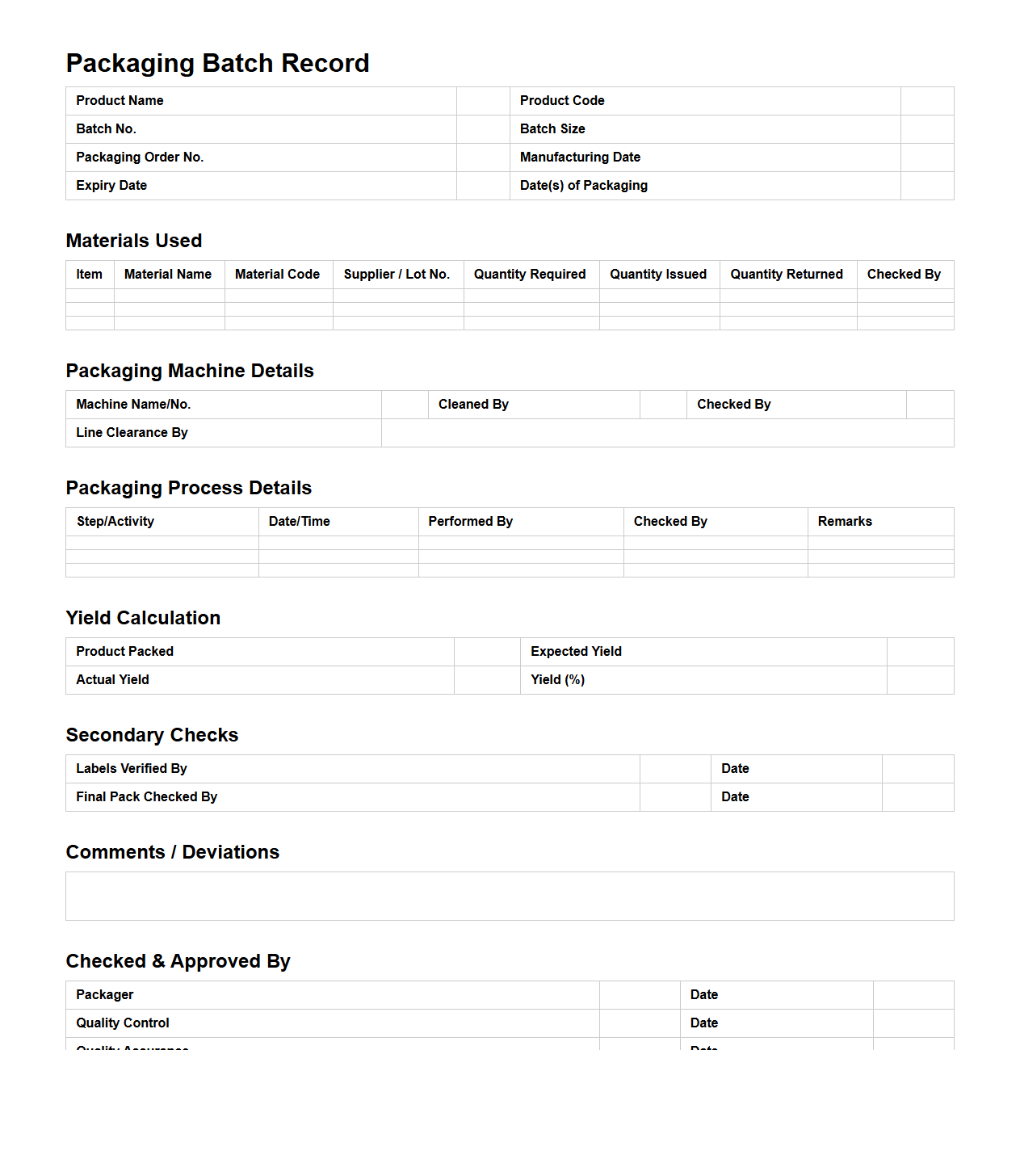
Packaging Batch Record for Pharmaceutical Products
A
Packaging Batch Record for pharmaceutical products is a detailed document that outlines the specific procedures, materials, and equipment used during the packaging process of a particular batch. It ensures traceability, quality control, and compliance with regulatory standards by recording critical parameters such as batch numbers, packaging materials, and operator details. This document is essential for verifying that each batch meets safety and efficacy requirements before distribution.
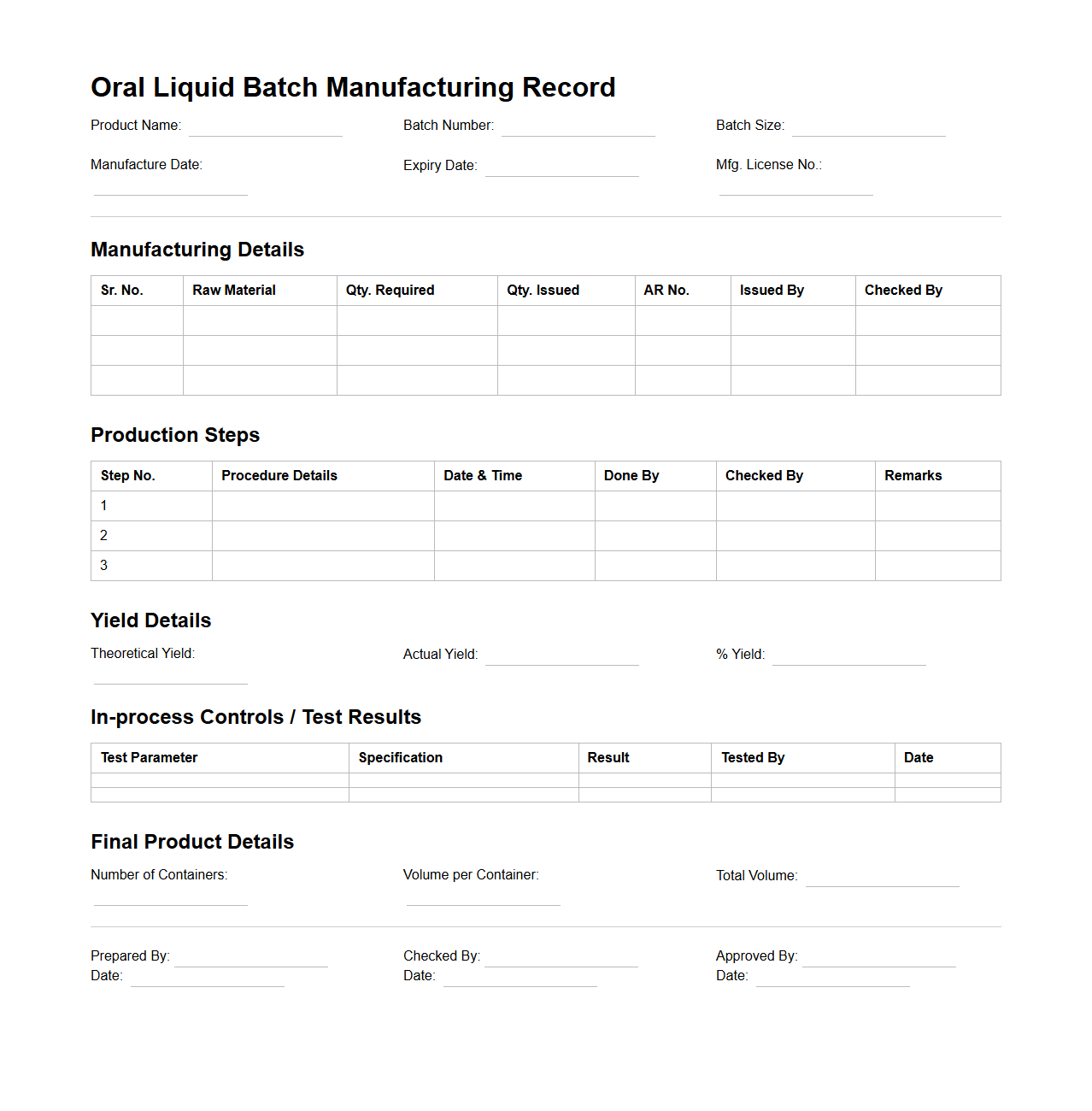
Oral Liquid Batch Manufacturing Record Example
An
Oral Liquid Batch Manufacturing Record Example document serves as a comprehensive template detailing the step-by-step production process of oral liquid pharmaceuticals, ensuring consistency and compliance with regulatory standards. It includes critical data such as ingredient quantities, equipment used, processing parameters, and quality control checks to maintain product integrity. This record is essential for traceability, auditing, and validating each batch produced in a pharmaceutical manufacturing setting.
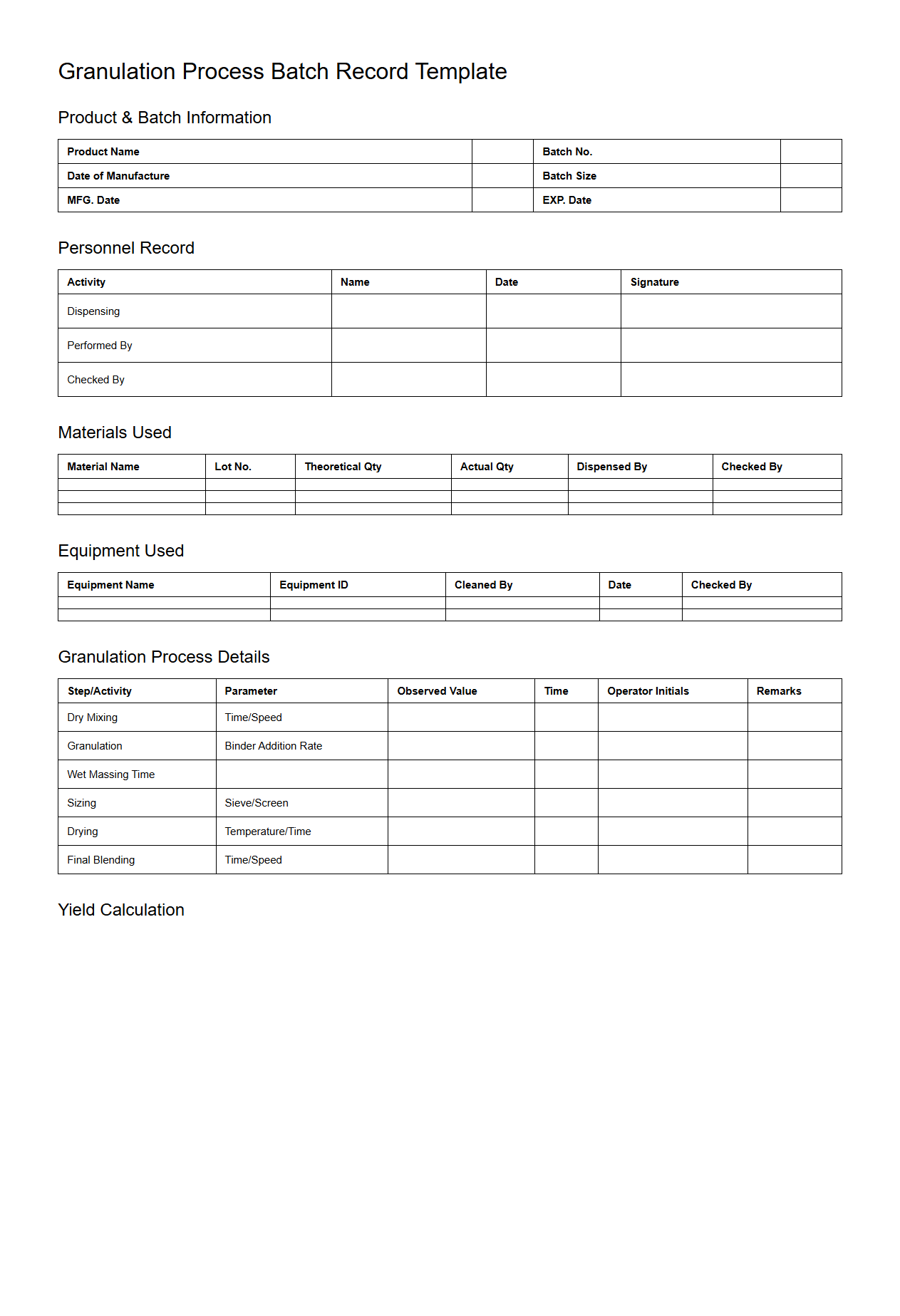
Granulation Process Batch Record Template
A
Granulation Process Batch Record Template document serves as a detailed guide for recording each step in the granulation manufacturing process, ensuring consistency and compliance with regulatory standards. This template captures critical data such as material lot numbers, process parameters, equipment used, and in-process quality checks to maintain product quality. It is essential for traceability, process validation, and audit preparedness in pharmaceutical and related industries.
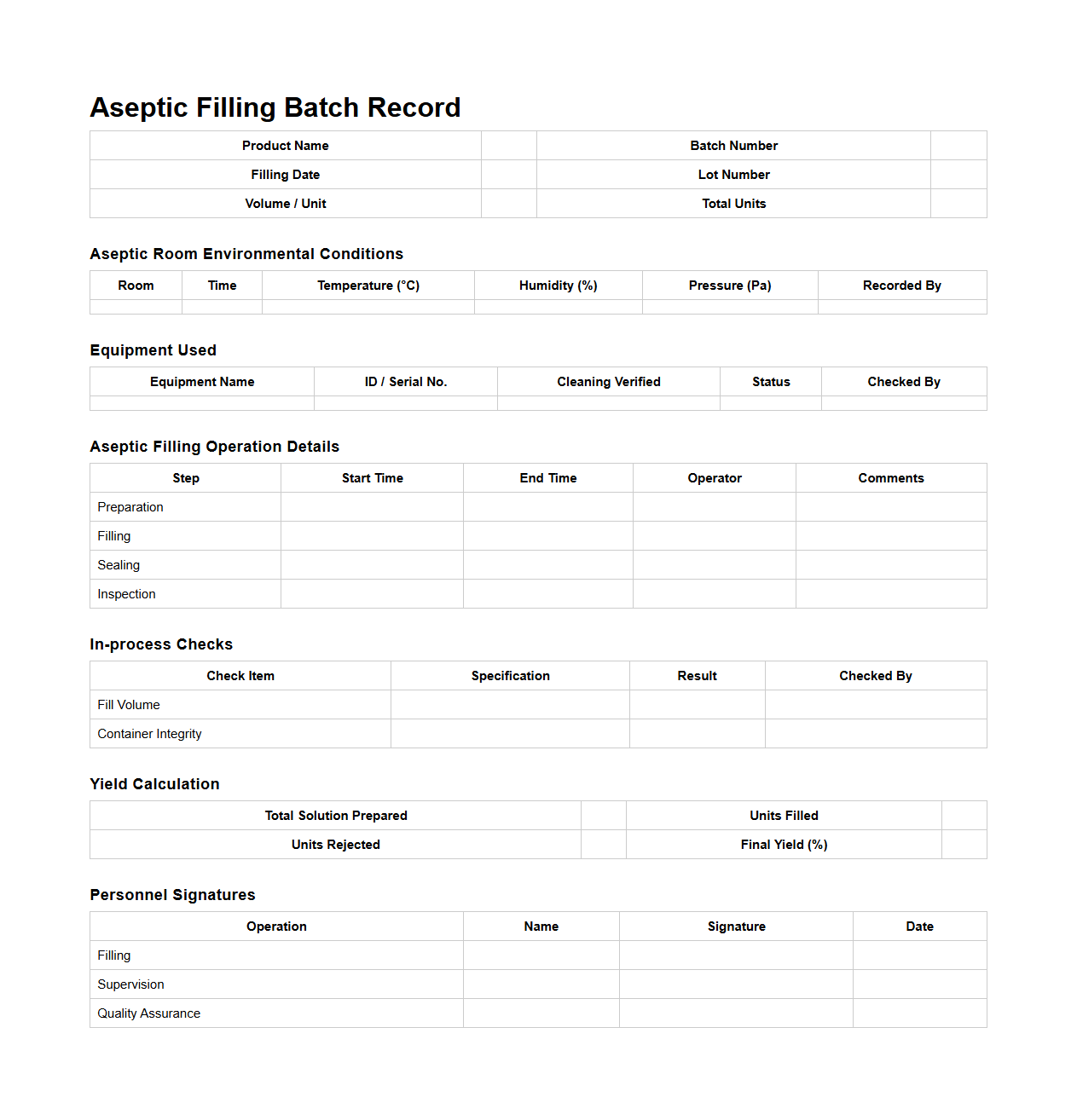
Aseptic Filling Batch Record Sample
The
Aseptic Filling Batch Record Sample document is a detailed template used to systematically document the entire process of aseptic filling in pharmaceutical manufacturing. This record ensures compliance with Good Manufacturing Practices (GMP) by capturing critical data such as equipment settings, environmental conditions, operator actions, and batch-specific details to maintain product sterility. It serves as a crucial reference for traceability, quality control, and regulatory inspections.
Active Ingredient Batch Manufacturing Record
The
Active Ingredient Batch Manufacturing Record document is a detailed record used in pharmaceutical manufacturing to track the production of active pharmaceutical ingredients (APIs). It ensures compliance with Good Manufacturing Practices (GMP) by documenting each step, including raw materials, processing parameters, equipment used, and quality control tests. This record is crucial for traceability, quality assurance, and regulatory audits.
Quality Control Batch Analysis Record
A
Quality Control Batch Analysis Record document is a detailed record that systematically captures the testing results and observations of a specific production batch in manufacturing. It ensures product consistency, compliance with regulatory standards, and traceability by documenting all quality control tests performed, including raw material identification, in-process checks, and final product evaluation. This document is critical for validating that each batch meets predefined specifications and is safe for distribution.
Equipment Cleaning Batch Record Form
The
Equipment Cleaning Batch Record Form is a critical document used in manufacturing and laboratory settings to systematically capture and verify the cleaning process of equipment between production batches. It ensures compliance with hygiene standards and regulatory requirements by documenting cleaning procedures, responsible personnel, cleaning agents, and inspection results. Maintaining accurate records through this form helps prevent cross-contamination and supports traceability during quality audits.
What critical information must be included in a Batch Record Document for pharmaceutical manufacturing compliance?
The Batch Record Document must contain detailed information about the raw materials used, including lot numbers and quantities. It should also document every step of the manufacturing process with precise instructions and outcomes. Additionally, it must include data on equipment settings, environmental conditions, and operator details to ensure full compliance.
Which sections of the Batch Record Document ensure traceability of materials and processing steps?
The sections covering material identification and batch numbers provide traceability for all raw materials used. The processing steps section records detailed actions, timestamps, and personnel involved to ensure accountability. Equipment logs and control parameters further enhance traceability throughout the manufacturing process.
How does the Batch Record Document facilitate deviation tracking and corrective actions during batch production?
The Batch Record Document includes dedicated sections for documenting any deviations from standard procedures during production. It requires operators to log the nature of deviations, potential impacts, and immediate corrections taken. This structure promotes transparency and supports systematic implementation of corrective and preventive actions (CAPA).
What data entries are mandatory for ensuring Good Manufacturing Practice (GMP) adherence in the Batch Record?
Mandatory data entries for GMP compliance include batch identification, raw material specifications, and process parameters. Operators must sign and date each recorded step to verify accuracy and accountability. Reporting environmental conditions and equipment calibration status is also crucial to meet GMP standards.
How does the Batch Record Document support batch review and release decisions by quality assurance?
The Batch Record Document provides a comprehensive summary of the manufacturing process, allowing quality assurance to verify compliance with predefined criteria. It includes all analytical test results and documentation of any deviations or corrective actions taken. This complete and traceable data set enables informed decisions regarding batch release or rejection.
More Manufacturing Templates