A Non-Conformance Report Document Sample for Quality Assurance is a crucial tool used to identify, document, and address deviations from established quality standards during production or service processes. It helps organizations systematically analyze defects or non-compliances, ensuring corrective actions are implemented to prevent recurrence. This structured report enhances overall quality management by providing clear evidence and accountability in quality assurance practices.
Non-Conformance Report Template for Manufacturing Quality
A
Non-Conformance Report Template for manufacturing quality is a standardized document used to identify, document, and track deviations from specified product or process standards. It captures critical information such as the nature of the non-conformance, root cause analysis, corrective actions, and responsible personnel to ensure effective resolution and continuous improvement. This template enhances quality control processes by providing a consistent approach to managing defects and maintaining compliance with industry regulations.
Quality Assurance Non-Conformance Investigation Form
The
Quality Assurance Non-Conformance Investigation Form document is a critical tool used to identify, document, and analyze deviations from established quality standards in manufacturing or service processes. It captures detailed information about the non-conformance, including root cause analysis, corrective actions, and responsible personnel to ensure effective resolution and prevent recurrence. This form helps maintain compliance with industry regulations and supports continuous improvement within quality management systems.
Product Non-Conformance Incident Report Example
A
Product Non-Conformance Incident Report example document provides a structured template to record details of a product that fails to meet specified quality standards or regulatory requirements. It typically includes fields for identifying the non-conforming product, describing the nature of the defect, root cause analysis, and corrective actions. This document is essential for maintaining quality control and ensuring continuous improvement within manufacturing or production processes.
Supplier Non-Conformance Notification Document
A
Supplier Non-Conformance Notification Document is a formal record used to report discrepancies or deviations in supplied materials or services that fail to meet specified quality standards. It details the nature of the non-conformance, including the affected batch or shipment, descriptions of issues, and any immediate corrective actions required. This document serves as a critical communication tool between buyers and suppliers to facilitate timely resolution and prevent recurrence of quality defects.
Process Non-Conformance Record for Quality Control
A
Process Non-Conformance Record is a crucial Quality Control document used to identify, document, and track deviations from established manufacturing or operational processes. It captures detailed information about the nature of the non-conformance, its root causes, and corrective actions taken to prevent recurrence. This record supports continuous improvement efforts and ensures compliance with industry standards and regulatory requirements.
Equipment Non-Conformance Documentation Sample
An
Equipment Non-Conformance Documentation Sample is a detailed record used to identify, document, and track deviations or defects in equipment performance or specifications. It serves as a standardized form to capture essential information such as the nature of non-conformance, root cause analysis, corrective actions, and responsible personnel. This document is critical for quality control and regulatory compliance in manufacturing, engineering, and maintenance processes.
ISO 9001 Non-Conformance Log Template
The
ISO 9001 Non-Conformance Log Template document is a systematic tool designed to record and track instances where products, processes, or services fail to meet specified quality standards. This template facilitates the identification, documentation, and management of non-conformities, enabling organizations to implement corrective actions effectively. Maintaining this log is crucial for continuous improvement and compliance with ISO 9001 quality management system requirements.
Construction Site Non-Conformance Report Sample
A
Construction Site Non-Conformance Report (NCR) Sample document provides a standardized template for identifying, documenting, and addressing deviations from project specifications, safety standards, or quality requirements on a construction site. It includes detailed information such as the nature of the non-conformance, responsible personnel, corrective actions required, and timelines for resolution. This report ensures systematic tracking and resolution of issues to maintain compliance and uphold project integrity.
Laboratory Non-Conformance Statement Form
The
Laboratory Non-Conformance Statement Form document records deviations from established laboratory protocols, procedures, or standards during testing or experimentation. It serves as a formal record to identify, analyze, and address non-conformities to ensure accuracy and compliance in laboratory operations. Maintaining this form helps laboratories implement corrective actions and continuous improvement measures, enhancing overall quality control.
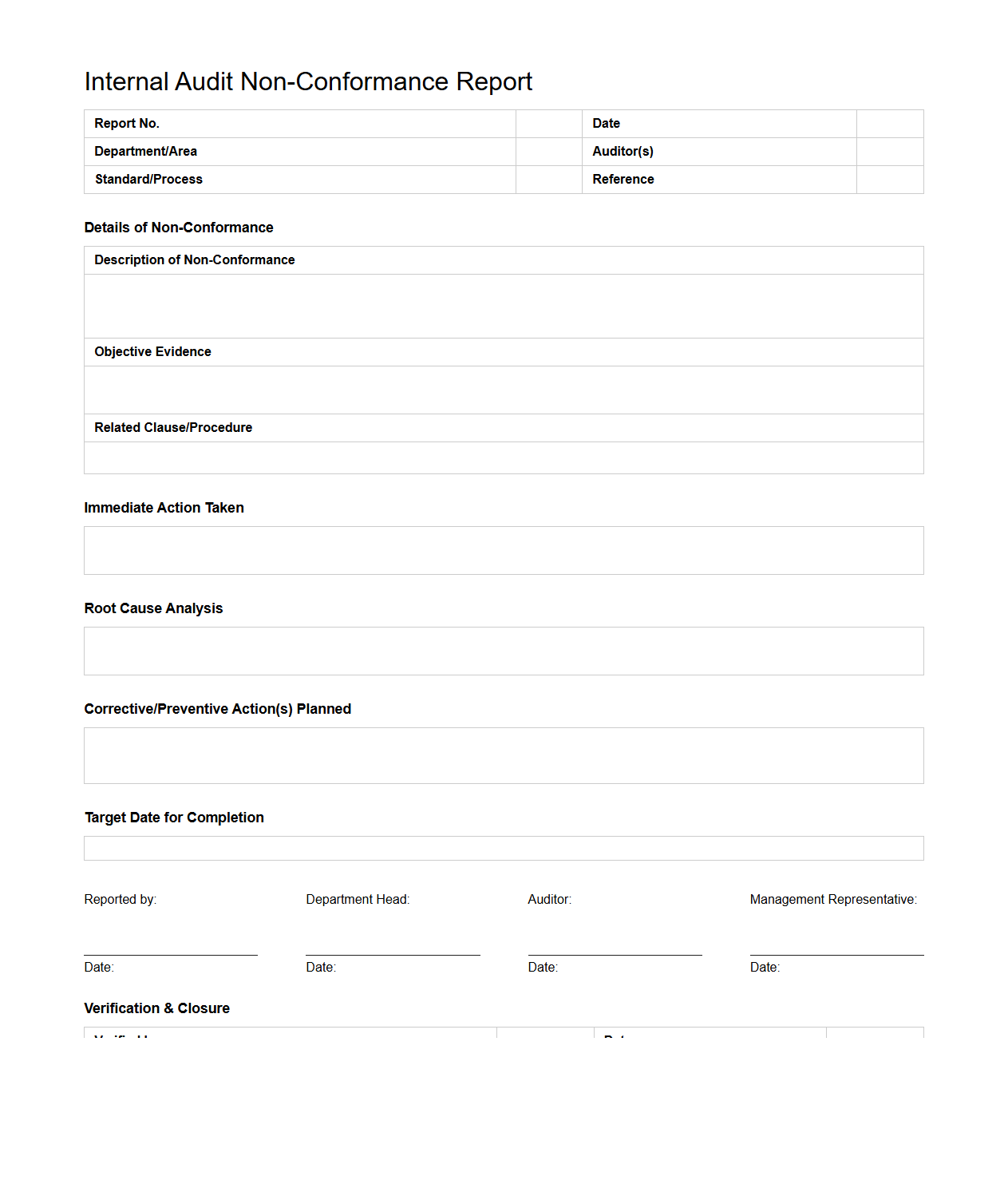
Internal Audit Non-Conformance Report Format
The
Internal Audit Non-Conformance Report Format document serves as a structured tool to record deviations or non-compliance identified during internal audits. It captures critical information such as the description of the non-conformance, its impact on processes or standards, root cause analysis, and recommended corrective actions. This format ensures consistent reporting, facilitates effective communication of audit findings, and supports continuous improvement within organizational quality management systems.
Essential Information in a Non-Conformance Report (NCR) for Traceability
A Non-Conformance Report (NCR) must include detailed identification data such as the NCR number, date, and the responsible department to ensure effective traceability. It also requires a description of the non-conformance, including the affected product or process, to provide context. Additionally, the report should document the origin and detection points to track the issue throughout the workflow.
Capturing the Root Cause in the NCR Sample
The NCR sample captures the root cause by using systematic analysis methods such as the 5 Whys or Fishbone Diagram explicitly documented in the report. It includes a section where investigators describe findings that led to identifying underlying reasons for the non-conformance. This structured approach ensures the root cause is clearly linked to corrective actions.
Assigning and Tracking Corrective Actions and Responsibilities
The NCR document assigns corrective actions by specifying responsible individuals or departments along with deadlines for completion. It features a tracking table or log that monitors progress and records updates on the implementation status. This transparency ensures accountability and timely resolution of issues.
Documenting and Communicating Impact Assessment
The impact assessment of the non-conformance is documented through an evaluation of the severity, scope, and potential risks associated with the issue in the NCR. This assessment is communicated by summarizing how the non-conformance affects product quality, safety, or compliance standards. Clear communication ensures stakeholders understand the implications and prioritize response measures.
Verifying Closure and Effectiveness of Corrective Actions
The NCR sample verifies closure by requiring documented evidence such as test results, inspections, or audits confirming that corrective actions were completed. It includes an effectiveness review section where results are analyzed to determine if the non-conformance has been fully resolved. This method ensures continuous improvement and prevents recurrence.
More Manufacturing Templates